Authors: Steve Bowditch, Jorge Mellado.
This paper was presented at the Corrosion & Prevention 2023.
ABSTRACT
Suitable protective surface coating can significantly extend the working life of components within wastewater systems. However, because elements and materials in such systems are subjected to enormous environmental stresses, choosing an appropriate coating for each can pose a challenge. One solution is to better understand the types of corrosion that various parts are prone to and identify key performance indicators (KPIs) to simplify the selection of potential surface coatings. Additional factors such as health and safety, cost, and technical support requirements may impact the final decision. However, a product that meets the KPIs is essential for engineering and operational efficiency. This paper will evaluate the various forms of corrosion impacting water systems in potable and wastewater systems. Classification of primary corrosion forms, including examples and root causes, will illustrate the impact on surfaces – uncoated or coated – with incompatible technology. Examples of compatible coatings will offer possible solutions and an overview of often overlooked KPI factors to maximise systemic longevity.
Keywords: water, wastewater, corrosion, protective coatings, surface coatings
Introduction
Wastewater facilities are critical for a functional society. They manage, treat, and make wastewater safe, allowing the reuse of this vital resource. Reliable water reclamation protects the environment from unnecessary contamination from domestic and industrial use.
Population growth is an exponential expectation throughout the Asia Pacific region, with significant investment required to upgrade and expand public wastewater facilities. This demand must also balance the societal need for greater environmental protection, leading to increased regulation over privately operated facilities. Industrial wastewater facilities are subjected to more stringent oversight to avoid overflows, leaks, and cross-contamination. Older plants must raise their standards and improve efficiency or face closure.
Plant operators can ensure compliance and longevity by increasing their knowledge of common corrosion issues. Of paramount concern is understanding what contributes to failures and preventative protective coatings to stop them. This is useful for every stage of plant life, including commissioning, maintenance, repair, and replacement.
This paper addresses common corrosion issues in wastewater plants and provides pictorial examples of their outcomes. It also offers KPIs for protective coatings that inhibit each type of failure. Equipped with the facts, individual plant operators can resource an appropriate coating specialist and proactively manage corrosion and related failures before they occur.
Discussion
This paper will provide a valuable guide for businesses working to create or improve their protective coatings strategy. Focus has been given to corrosion mechanisms most commonly affecting process equipment such as screens, clarifier rake arms, de-canters, pumps, valves, and mixers, as well as structures such as clarifiers, bar screen chambers, thickeners, manholes junction boxes and wet well/lift stations. Protective coating that meets KPIs to address these situations will then be explored.
Corrosion of Metals – Pitting Corrosion
Pitting corrosion (see Figure 1) occurs on carbon steel and cast-iron alloys when the passivated natural oxide (Fe2O3) layer on a metallic surface is disturbed. This results in the exposed region assuming an anodic (low pH) reaction while the covered areas remain cathodic. As the anodic region often has a smaller surface area than the cathodic region, corrosion ‘pitting’ commences in the anodic region. As deterioration progresses, the pit deepens, and the pH levels drop, causing the anodic/cathodic reaction to continue, albeit faster. Eventually, the cavity will exceed the vessel wall thickness, increasing the risk of leaks and structural failure.
Stainless steel does not form oxide layers like carbon steel or cast iron. However, it is still prone to pitting corrosion under specific circumstances, such as in the presence of oxygen or oxygenated water flow. Stainless steels containing chromium at a level >11% form a passive layer of chromium oxide, and this layer prevents corrosion. If the surface has a biofilm cover, a gasket connection, or fabrication detail that create an area of low/no flow, oxygen levels in these regions reduce. These oxygen-depleted zones become anodic, and corrosion commences.
Chloride levels can further exacerbate pitting corrosion rates on stainless steel, including when using ferric chloride (FeCl3) as a flocculating agent or to reduce sulphides. Type 304 and 316 stainless are prone to excessive pitting corrosion with chloride levels >300 ppm, even in neutral pH solutions.
Aluminium is a thermodynamically reactive metal, so it is prone to corrosion compared to noble metals. Within pH ranges of 4.5-8.5, aluminium is corrosion resistant, but in enclosed head spaces with wastewater exposures where hydrogen sulphide gases build up and result in pH <4, pitting corrosion can occur on the aluminium oxide layer.
KPIs of Coatings Intended for Pitting Corrosion Exposures:
- High tensile adhesion (>20 MPa) to resist under-film corrosion
- High abrasion resistance to avoid exposing regions that can become anodic (tensile strength >40 MPa to ASTM D638 and cathodic disbondment <2 mm to ASTM G8)
- Low permeability (water vapour transmission <200grams/m2 over 24 hrs to ASTM E96)
- General resistance to thermal and chemical exposure
Therefore, a coating to be applied to protect against this type of corrosion must have the above specifications at a minimum.

Corrosion of Metals – Graphitic Corrosion
Commonly seen in cast iron and ductile iron when buried in acidic soils or exposed to mildly acidic waters, Graphitic corrosion (See Figure 2) depends on the acidity where the soil pipes are buried. Further influences include soil moisture content and any contamination. Under these conditions, the iron (Fe) leaches out of the casting into the soil or water flows, leaving behind a weakened matrix of graphite and iron oxides. Casual observation will not indicate any damage, but the remaining matrix of graphite and iron oxides is weak and soft and, if left unaddressed, can lead to collapse or structural failure. Corrosion progresses slowly, so affected systems may not show the extent of the deterioration for decades. Many international cities have cast and ductile iron pipes, creating ongoing issues. Upgrade efforts of the pipework present an uphill struggle that is hindered further by the silent creep of this form of corrosion.

KPI’s of Coatings Intended for Graphitic Corrosion Exposures:
- High tensile adhesion (>15 MPa) to better resist under film corrosion
- High tensile properties for resistance to soil shear stress
- Impact and abrasion resistance for buried pipe
- Low permeability
Corrosion of Metals – Galvanic Corrosion
Two dissimilar metals cause this form of corrosion when differing electromotive potentials come in contact with one another in the presence of a common electrolyte (see Figure 3). In this case, the less noble metal becomes anodic and goes into an ionic solution, sacrificing itself to protect its more noble cathode.
The rate and severity of corrosion losses relate to electromotive potential differences between the anode and cathode. The pH and conductivity of the electrolyte exposure, the two metals’ proximity to each other, and the relationship of the anodic region’s surface area compared to the cathodic all contribute to corrosion rates. If the anodic region is larger than the cathodic region, corrosion rates will be slower than if the anodic area is smaller.
Treatment plant operators must also be concerned with zinc galvanised surfaces that come in contact with unprotected carbon steel or aluminium structures, such as rubbish racks or gates. These might be electrically coupled to embedded reinforcing rods in the concrete with carbon steel anchor bolts and frames, resulting in corrosion.

KPI’s of Coatings Intended for Galvanic Corrosion Exposures:
- High tensile adhesion (>20 MPa) to resist under film corrosion
- High dielectric resistivity
- Resistance to cathodic disbondment
- Low permeability
- General resistance to thermal, chemical exposures
Corrosion of Metals – Microbiologically Influenced Corrosion (MIC)
MIC is commonly found in wastewater treatment collections and treatment facilities in both aerobic as well as anaerobic conditions. (see Figure 4) The primary form of MIC in wastewater treatment plants is biogenic sulfide corrosion which occurs when anaerobic sulfate reducing bacteria (SRB) metabolize the sulfate (SO2-4) ions which are rich in the untreated wastewater flows. The SRB’s metabolic process consumes oxygen, and the resulting by product is the sulfide ion (S2-). This ion is released into the water flows. The sulfide ions combine with hydrogen in the water flows to form hydrosulfide or bisulfide (HS-) ions. These ions react further to form hydrogen sulphide (H2S) which continues to build to saturation levels. In turbulent flows the H2S gas, as well as carbon dioxide (CO2) are released into the humid enclosed head spaces commonly seen in manholes, wet wells and lift stations, junction boxes, collection pipe, junction boxes and then into the plant in bar screen chamber, grit chambers as well as primary and secondary clarifiers. These gases combine with the humid atmosphere and form weak thiosulphuric and carbonic acid which depresses the surface pH. Once the pH drops below 9.5, naturally occurring sulfur oxidizing bacteria (SOB) can colonize on the surface and they in turn metabolize the H2S generating a weak sulphuric acid. This can result in surface pH <5. Mild steel and ductile iron, present in closed head spaces such as piping, valves, pumps, and associated structural members are then subject to accelerated corrosion rates from the acidic environment.

Corrosion of Concrete – Microbiologically Influenced Corrosion (MIC)
The same processes above that generate dilute sulfuric acid are present in enclosed head spaces of many wastewater concrete structures, such as manholes, wet well/lift stations, junction boxes, collection pipes (force and gravity flow), bar screen chambers clarifiers, and sludge tanks (see Figure 5). Unfortunately, corrosion is significantly faster and more dramatic in concrete due to its alkaline nature (12.5 when freshly poured). When reacting with the dilute sulfuric acid pH can easily reach <2. The acid reacts with and converts the cement calcium hydroxide to calcium sulfate salts, easily dissolved in water. This leads to the loss of cement paste and the release of aggregate and sand constituents in concrete, eventually exposing structures embedded into the concrete. The resulting surface can be exceptionally rough and difficult to coat with thin (<1 mm) coatings.
KPI’s of Coatings for Metal/Concrete Surfaces Intended for MIC Corrosion Exposures:
- High tensile adhesion (>20 MPa) to resist under film corrosion on steel
- Resistance to Low pH (10% H2SO4)
- Resurfacing and barrier topcoats with edge retentive capability to prevent pinholes in applied films when being applied on heavily pitted surfaces
- Low surface energy to reduce biofilm build-up

Corrosion of Concrete – Acid Attack
The same acid-base reaction occurs when process chemicals used in wastewater treatment plants encounter unprotected concrete. Chemical attack frequently occurs when sulfuric acid is present and used as a buffer or when ferric chloride and alum are utilised for flocculating agents. The area’s most prone to attack are often containment and dose mixing stations, with the result like a MIC attack. Initially, concrete etching will appear, progressing to cement paste breakdown and aggregate exposure. Rebar corrosion and spalling are inevitable if early warning signs are left unaddressed.
KPIs of Coatings for Concrete Surfaces Intended for Chemical Exposures:
- Resistance to low pH from concentrated and dilute (H2SO4, FeCl-, NaOH, NaOCl)
- Impact and abrasion resistance
- Low coefficient of thermal expansion for when thermal cycling exists
- Resurfacing and barrier topcoats with edge retentive capability to prevent pinholes in applied films when being applied on heavily pitted surfaces
KPI’s Defined
KPIs and those relevant to your specific application will always drive selection for coatings. As outlined in each KPI section above, the applied coating must be able to protect against the particular type of corrosion being experienced and relevant to the associated material specifications. KPI:
- Edge Retentive Properties
- High tensile adhesion
- Low permeability
- High abrasion resistance
- Resistance chemical exposure
- Low Surface Energy
Edge Retentive Properties (KPI)
Corrosion of a coated substrate typically starts where there is a sharp edge, a weld (see Figure 6) or where some surface irregularity such as pitting is present. Coatings with high edge retentive properties (see Figure 7) ensure complete coverage of irregular shapes and sharp edges. Coatings with high edge retention can provide sufficient coverage after only one coat, saving time and money on the application process and provide better protection to the substrate. Due to a coatings surface tension, it will tend to pull away from rough weld regions, edges, and angles.

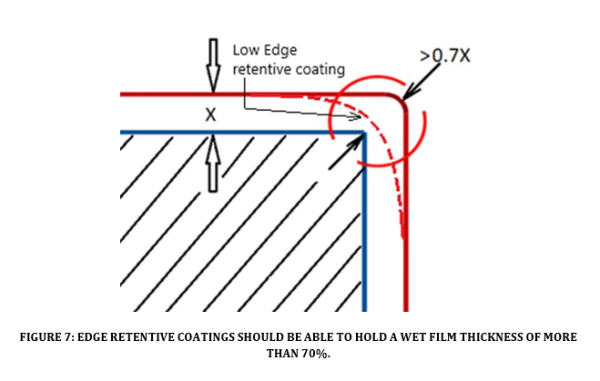
Sharp edges and angles are zones of high surface energy which hampers a films ability to “wet out” and adhere. This “pulling back” leads to reduced film thickness at the edge region that can become a corrosion initiation site (see Figure 8). Corrective actions include radius grinding, stripe coating and edge retentive coating adoption. An edge retentive coating is a thixotropic film with anti-sag properties that improves the retention of the coating around sharp edges. An edge retention coating can be applied in a continuous film over a sharp edge while maintaining >70% of the applied wet film thickness. This property ensures adequate coverage over edges and sharp angles. Properly applied edge retentive coatings lessen the reliance on radius grinding, stripe coating, and generally speeds up the application process.

High Tensile Adhesion (KPI)
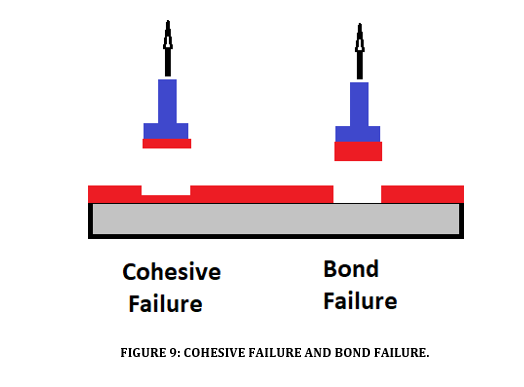
High tensile adhesion gives a coating the ability to resist delamination and prevent under-film corrosion (see Figure 9). A quality coating can have a tensile adhesion of more than 20 MPa which is far greater than the tensile strength of concrete, so physical removal of the coating by tensile force will typically mean that some of the concrete substrate will delaminate. A coating with low tensile adhesion can be susceptible to delamination or cohesive failure with impact. High tensile adhesion in the range >20 MPa is an accepted industry figure to resist delamination and under film corrosion.
Low Permeability (KPI)
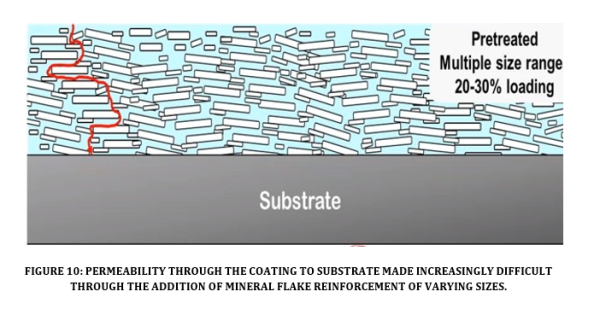
Permeability controls the passage of corrodents through the coating to the substrate (see Figure 10). A coating exhibiting high permeability will typically fail faster. Additional treating processes that prepare the surface of reinforcements in coatings and assist in developing powerful bonds to the surrounding epoxy, help to reduce permeability. Mineral reinforcements increase the barrier properties to prevent the permeation of both liquids and gases.
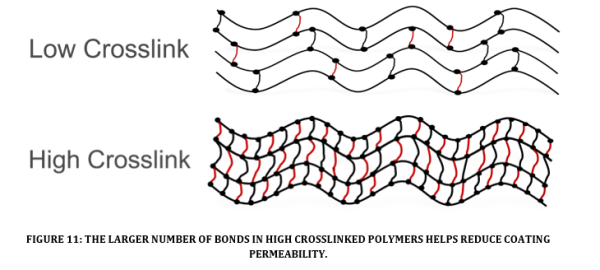
The formation of covalent bonds which hold polymer chains together is called crosslinking (see Figure 11). Low levels of crosslinking yields higher flexibility with lowered mechanical, thermal and chemical properties. High levels of crosslinking yields lower flexibility with higher mechanical, thermal and chemical properties. Highly crosslinked polymers exhibit lower levels of permeability that help to retard the flow of corrodents from the surface to the substrate.
High Abrasion and Chemical Resistance (KPI)
High abrasion and chemical resistance are vital as wastewater flows can contain substantial abrasives that wear away surfaces. The coatings must have abrasion-resistant components while also withstanding splashing chemicals or total immersion where biogenic corrosion mechanisms are at play. It is pointless if the coating applied itself does not have a high degree of chemical resistance. Chemists and materials engineering specialists at reputable coatings manufacturers work hard to continually develop coatings with ever increasing levels of chemical resistance.
Polymer resins on their own do not exhibit high levels of abrasion resistance. Coating manufacturers employ the use of chemically resistant mineral reinforcements to improve a coatings resistance to abrasive flows. Key to ensuring the abrasion resistant media does what it’s meant to do, is its ability to adhere within the resin and maintain that barrier to the base resin. One way to achieve this is to use varying sizes of ceramic beads to interlock in a matrix. Ensuring the right loading and sizing of ceramic beads is then critical to achieving this. Another important factor is to ensure that the ceramic beads themselves are not easily dislodged from the base resin. Preparing beads by pre-treating them with coupling agents (see Figure 12) is very important in achieving a strong bond between the bead and the resin, allowing the beads to stay in place and do the job they are meant to do.
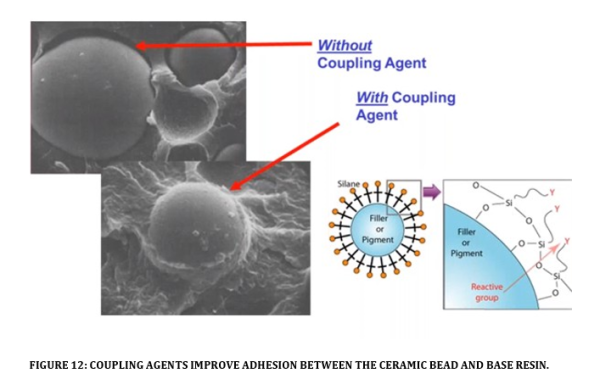
Without pre-treatment, beads can and do dislodge from the polymer resins, allowing for fast erosion. Ensuring the matrix of beads is maximised and the beads are held in place has the added benefit of reducing the permeability of the coating system.
Low Surface Energy (KPI)
One method of minimising biogenic corrosion is to simply regularly remove it from a surface. Once surfaces are damaged however, it is tough to keep them clean, made more challenging by inaccessibility. Coatings with low surface energy achieve high gloss levels (smooth surface finish) by managing the quartz flake reinforcement and resin viscosity. The resulting surface exhibits low surface energy characteristics that retard the formation of a biosolid layer (see Figure 13).

RECOMMENDED STEPS
Speak to an expert. The coating selection may still be a complex choice depending on the environment in which it will be used. The best way to identify which coating suits your application requirement is to consult an expert who can guide your requirements.
CONCLUSION
The information will assist corrosion-type identification and provide insight into available solutions. By understanding common types of corrosion and protective coating KPI requirements, operators can make informed decisions for their facility’s needs.
ACKNOWLEDGEMENTS
The authors wish to thank NACE instructor and mentor Lou Vincent, who taught that fighting corrosion is a worthwhile but never-ending battle.
REFERENCES
- https://infrastructurereportcard.org/cat-item/wastewater/
- Redner, R.P. Hsi, E. Esfandi, “Evaluating Coatings for Concrete in Wastewater Facilities: An Update”, Journal of Protective Coatings and Linings, December 1994; pp. 48-59.
- Jose Villalobos; Graham Bell, Chapter 13, “Corrosion in Wastewater Systems” Volume 13C; Corrosion: Environments and Industries; ASM International; 2006 https://doi.org/10.31399/asm.hb.v13c.9781627081849