Authors: N Davison, D Bewley, S Wheelhouse, A C Roberts, G K Glass.* ,
This paper was presented at the Corrosion & Prevention 2023.
Concrete Preservation Technologies, Long Eaton, Nottinghamshire, UK
*Corresponding author email: garethg@cp-tech.co.uk
ABSTRACT
Discrete galvanic anodes for concrete structures were developed to prevent corrosion from initiating on steel in concrete adjacent to an area of patch repair. Such corrosion is known as incipient anode formation. The UK National Highways Works Manual requires that acceptable galvanic anode products perform “without evidence of corroding reinforcement within concrete adjacent to and up to 300mm away from the repair perimeter”. The earliest method used to demonstrate this protection was potential mapping in about 1998. Another less sensitive but sometimes more accessible method is visual assessment. This work looks at the performance of aged structures where galvanic anodes have been used to address the incipient anode effect. Performance is evaluated using both potentials and visual assessment.
Keywords: Concrete, Galvanic Anode, Corrosion, Repair
Introduction
Corrosion is well understood to be a major cause of reinforced concrete deterioration. The first line of defence is to provide barriers to the ingress of the aggressive agents that cause corrosion. Adequate concrete cover, the use of high performance concrete and the application of coatings and sealants can all play a part in protecting the embedded steel reinforcement.
Nevertheless many structures are exhibiting corrosion damage within their design lives. Repairing corrosion damaged concrete involves removing the damaged concrete, cleaning the exposed steel reinforcement and restoring the concrete profile with a repair mortar. In many cases further corrosion induced deterioration has been observed to subsequently occur in the parent concrete in the immediate area around the patch repairs. This phenomenon is known as the incipient anode effect. Several possible mechanisms arising from the process of repairing the concrete may trigger the incipient anode effect [1].
At this stage in the life cycle, an appropriate corrosion control system may be used to keep a structure safe, maintain functionality and extend life. One of those systems involves the use of galvanic anodes. This work considers the performance and product acceptance criteria of such systems in reinforced concrete repair.
Galvanic Technology
Galvanic anodes are easily corroded metals (electrochemically active metals) often consisting of zinc that are connected to steel to corrode in preference to steel. A voltage difference between the anode and the steel causes a protection current to flow to the steel. This changes the steel potential. Further corrosion of the steel is inhibited. The steel becomes the cathode of the anode-steel connected couple.
Galvanic anodes are voltage limited devices. The protection current output is dependent on both the available voltage and the resistance of the environment. Because concrete generally provides a high resistance to current flow, galvanic anodes were initially only considered suitable to protect reinforcing steel in warm wet concrete environments where the concrete resistance was lower [2]. It was suggested that in more resistive concrete, the limited voltage of the anodes would limit any effect they might have on corrosion.
In the 1990’s proponents of galvanic technology suggested it could be used to address the incipient anode effect around patch repairs [3, 4]. Early methods of assessment involved the use of potential mapping [4]. In 2004, the responsive behaviour of galvanic anodes was noted (see Figure 1 below). Galvanic anode protection current responded to changes in environment resistivity in the same way that reinforcement corrosion responded to changes in environment resistivity [5]. More protection current was delivered in more aggressive conditions.
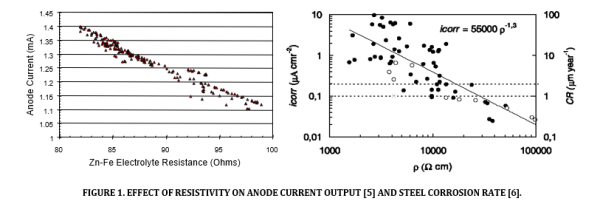
Further studies on the responsive behaviour of galvanic anodes improved the understanding of how they operate [7]. These studies confirmed that galvanic anodes are unlikely to deliver a sufficient steel potential shift to stop corrosion in high resistivity environments. However under laboratory conditions using equivalent zinc and steel anodes, it was noted that a zinc anode responds to environmental events that reduce the concrete resistance and increase the corrosion risk, such as moisture ingress and temperature increases, more rapidly than a steel anode. This means that the protective current being passed to the steel is increased just prior to an increased corrosion risk.
By 2012 several manufacturers had introduced galvanic anodes for use in concrete patch repair onto the market. They were marketed as ‘no monitoring and maintenance’ products with a life of at least 10 years. They have been used as standalone products, as well as in conjunction with reinforcement or concrete coatings. New arrangements were developed to promote the flow of current to the steel outside the area of patch repair [8]. The commercial justification for their use arises from the protection they deliver just outside the area of repair. This effectively means the repair covers a larger area. This is illustrated in Figure 2 below.

In 2020, the UK National Highways included the possible use of these products into their Manual of Contract Documents for Highway Works, Volume 1 Specification for Highway Works as Series 5700 – Concrete Repairs. A guidance document (Series NG5700) accompanied the specification [9,10]. The National Highways standard requires (clause 5712) under the heading “Acceptance of Products”:
Galvanic anodes … shall have a proven successful performance in service of at least five years on similar structures, with comparable environmental exposure. The Contractor shall demonstrate this by providing examples of installations where the proposed anode has performed satisfactorily in repair patches without evidence of corroding reinforcement within concrete adjacent to and up to 300mm away from the repair perimeter.
Thus for a product to be acceptable, at least two examples from similar structures with comparable environmental exposure must be provided showing no evidence of reinforcement corrosion up to 300mm away from the perimeter of the concrete repairs after 5 years. That evidence might for example be provided by visual assessment.
The standard requires under the heading “Electrical Potential Survey”:
A survey of electrical potential shall be undertaken by the Contractor on the surface of the existing concrete outside the repair area. Survey points shall be located 250mm outside the perimeter of the repair area, and shall be spaced 500mm apart. The electrical potential survey shall be done after removal of defective concrete, but before galvanic anodes are attached to the reinforcement. The method of survey shall comply with ASTM C876.
The electrical potential survey shall be repeated by the Contractor after completion of the repair using the same potential survey instrument with new readings taken at the same survey grid points. The repair concrete should be at least 28 days old.
This might be more aptly named a steel potential survey. An example of before and after steel potentials recorded at an area of concrete repair to a highways agency structure in 2023 is provided in Figure 3.
Potential measurements may be used as a form of commissioning. The data can show that the anodes are connected and are providing protection outside representative patch repairs when they are installed. The use of the technology does not otherwise place any additional monitoring or maintenance requirements on the repaired structure outside of the visual inspection required by regular structural inspections.

Case Histories
Three case histories are provided in this section. The first two used both potential mapping and visual assessment to assess performance. The third is not strictly a galvanic patch repair but it included the use of other methods to assess galvanic anode response and performance.
Grosvenor Car Park
Grosvenor car park was constructed around 1975. By 2010 it had suffered extensive corrosion damage to the decks as a result of chloride contamination from de-icing salts. This resulted in visible cracking and spalling on all decks (Figure 4). Repair of the structure commenced at the end of 2010.

The car park had previously been repaired and was also the site of an early galvanic anode trial installed in about 1999 which was now showing signs of deterioration in the form of cracks running across the repair/parent concrete interface. The client had also had some experience of the complexities of an impressed current system installed on another nearby car park, but this was not a positive experience. All electrochemical treatments have limitations. The issues are lack of power, mainly affecting galvanic systems, or issues surrounding complexity, mainly affecting impressed current systems.
The solution for the Grosvenor car park included the use of galvanic anodes installed in drilled holes at the edge of the concrete repair. The anodes were connected to the steel exposed in the repair using a connector of the anode assembly (Figure 5). The application of a high quality repair mortar completed the repair process [11]. To reduce complexity there was no facility to disconnect the anodes or measure current output. The commissioning process for galvanic anodes was in its early stages of evolution at this point in time.

Performance assessment was conducted by mapping the potential on the surface of the concrete adjacent to the repaired area relative to a stationary electrode. This was used to determine the extent of protection provided by the anodes and check the anode spacing. An example of the data is provided in Figure 6. It shows the potential gradient caused by the decaying effect of the anodes in the area of patch repair as a function of the distance from the edge of the repair.
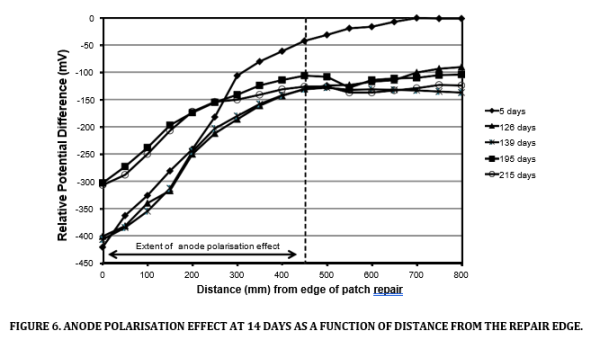
The polarisation effect was monitored over a period of 215 days at various locations throughout the car park. The distance of the effect ranged between 500 and 750mm from the edge of the repairs [10]. After this time the deck was coated and no longer accessible to potential mapping (Figure 7).

In 2023, more than 12 years after installation of the anodes, a visual inspection of the car park was undertaken. There was substantial evidence of ponding water and some evidence of coating wear, but no evidence of further corrosion induced damage was visible. Examples showing the condition of the car park are provided in Figures 8 and 9.

Milton Keynes Canal Bridge
Milton Keynes canal bridge supports a dual carriageway over a canal in the UK city of Milton Keynes. By 2011 the bridge abutment had suffered extensive chloride induced corrosion damage. The source of the chloride in the abutments arose from the use of road de-icing salts and a leaking expansion joint above the abutment. The solution included the use of galvanic anodes in the patch repairs. Photos showing the extent of the repair and the location of some anodes are provided in Figure 10. Anodes were also installed on a grid pattern in drilled holes outside the repairs.

A photo showing the completed installation in 2011 is provided in Figure 11.

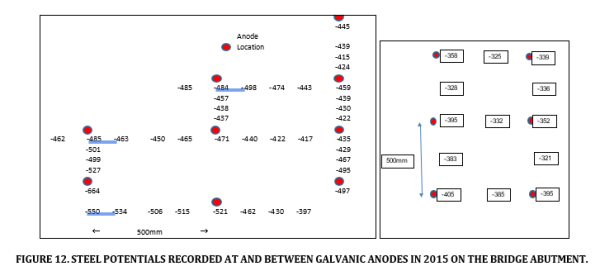
No potential mapping data from 2011 was available for this structure as the process of commissioning these systems was still evolving. However a site visit in 2015 did record some steel potential data at and between the installed anodes at two locations. This is provided in Figure 12 for two representative locations. The anodes are installed in a grid pattern at 500mm centres and the steel potentials at the anodes were distinctly more negative than the steel potentials between the anodes.
A brief site visit was undertaken in 2023. A general overview of the visible condition of the abutment is provided in Figure 13. There were no visible signs of corrosion.

Whiteadder Bridge
By February 2007, the piers of Whiteadder Bridge, near Berwick-upon-Tweed in Northumberland had suffered extensive visible cracking and delamination of the concrete cover as the result of the use of de-icing salts. Chloride contamination was high at up to 1.68% by weight of cement and the piers were subject to extreme wet–dry cycling in a flood plain. The damage is illustrated in Figure 14.

A hybrid galvanic corrosion protection system was installed on the piers as part of the solution. This installation was not strictly a galvanic system. An initial temporary impressed current treatment was applied using the anodes. The system included reference electrodes and both steel potentials and currents have been monitored since installation. The temporary impressed current was used to arrest the ongoing corrosion in the aggressive conditions experienced by this structure.
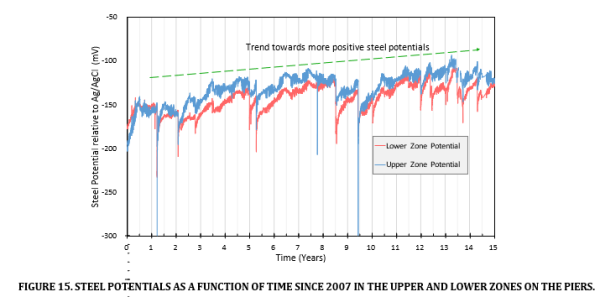
The average steel potential for both zones, following the 2007 repair, is plotted as a function of time in Figure 15. The trend is towards more positive or passive steel potentials. The temporary negative shifts in steel potential are associated with a substantial increase in current output which was a consequence of heavy rainfall and flooding [12].
The trend in current is provided on a logarithmic scale from years 7 to 16 in Figure 16 below. The trend in current has not substantially changed over this period. The biggest effect on current output are the flooding events which give rise to an order of magnitude increase in current output.
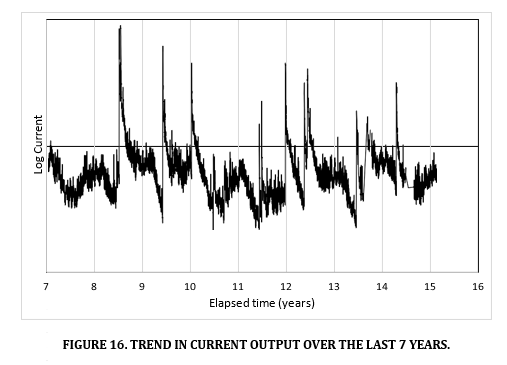
Current and potentials have been used to determine corrosion rates and in both zones the corrosion rates are indicative of passive steel. Higher currents were measured in the lower zone, but steel passivity was stronger in the dryer (more resistive) upper zone (corrosion rates were lower and corrosion potentials were more positive) [13,14].
In 2023 a visual inspection of the structure was undertaken and no visible signs of corrosion induced deterioration were identified [13]. A general view of the structure in March 2023 is provided in Figure 17.

Other workers have suggested that galvanic current output should be used to rank and select appropriate products at the design stage. Indeed using the current outputs previously reported for the Whiteadder bridge, they predicted that this repair system would have been unacceptable by 2013 [15]. However, in reality, the 2007 repair is showing no signs of corrosion distress in 2023. The system at Whiteadder has outperformed the 1999 galvanic anode trial installed on the Grosvenor car park (cited above). The anode arrangement employed at Whiteadder resulted in the current being strongly dependent on the environment via its effect on concrete resistivity.
By contrast, the anodes in the 1999 trials were encased in a highly conductive mortar to form an assembly that was tied directly on to the steel. The anode assembly and the steel were then encased in a more resistive repair material. The highly conductive mortar may provide a path for high currents to flow directly to the local steel in the repair area, causing unnecessary polarisation of the anode. Both the unnecessary polarisation of the anode and the resistive repair mortar may then prevent the anode from achieving the desired effect on the steel 300mm outside the repaired area.
For these and other reasons, it is risky to use current data to select galvanic anodes for concrete repairs. Such a use of current output as a requirement for product acceptance would promote arrangements that dump large currents onto localised steel surfaces without delivering any substantial protective effect. Past performance based on visual assessment and steel potential measurements are the preferred methods of assessing product suitability that also fit with the maintenance free nature of the galvanic solution.
Observations and Conclusions
Many repair systems that rely on galvanic anodes have now provided protection to steel in concrete for more than a decade. Following installation and commissioning, these systems require no maintenance. They respond in a positive way to changes in the aggressive nature of the concrete environment which give rise to changes in concrete resistivity. There primary limitation is a lack of power. Monitoring does not extend beyond that already undertaken.
In 2020, UK National Highways provided a specification for concrete repairs using galvanic anodes for National Highways works. The specification sets out requirements for acceptable products and provides commissioning details. Commissioning involves the use of steel potential mapping and may be used to assess the effect on the steel in the parent concrete adjacent to the patch. Monitoring is limited to the visual assessment undertaken as part of the bridge inspection process.
Current output data is not suitable for predicting long term product performance. Galvanic anodes are voltage limited devices and protection current is determined by environment resistivity. Encasing the anode in a highly conductive mortar to form an assembly, then tying it directly on to the steel and embedding the anode assembly and steel in a highly resistive repair material may prevent the anode from achieving the desired protective effect on the steel outside the repaired area. Past performance based on visual assessment and steel potential measurements are the preferred methods of assessing product suitability.
References
- Christodoulou C, Goodier C, Austin S, Webb J and Glass GK, Corrosion Science, 69 (4) (2013) 123-
- BS EN 12696:2000, Cathodic protection of steel in concrete, British Standards Institute, (2000).
- Bartholomew JJ, Bennett JE, Martin BL and Mitchell TA, Method and Apparatus for Cathodically Protecting Reinforced Concrete Structures, US Patent Application No. 892913, June 1992.
- Page CL and Sergi G, Journal of Materials in Civil Engineering, 12(1), (2000) 8 –
- Glass GK and Davison N, “Long term performance of galvanic anodes in concrete” (Invited Presentation), TRB technical committee meeting on Concrete Durability, Transport Research Board, Washington, January 2004
- Morris W, Vico A, Vazquez M and de Sanchez SR, Corrosion Science, 44(1) (2002), 81-99
- Holmes SP, Wilcox GD, Robins PJ, Glass GK and Roberts AC, Corrosion Science, 53(10) (2011). 3450-
- Christodoulou C, Goodier CI and Austin SA, “Site performance of galvanic anodes in concrete repairs”. in: Grantham, M, et al (eds). Concrete Solutions 2014. Proceedings of Concrete Solutions, the 5th International Conference on Concrete Repair, 1st-3rd September 2014, Belfast, Boca Raton, Fl: CRC Press, pp. 167-172.
- MCHW Series 5700 – Concrete Repairs see: https://www.standardsforhighways.co.uk/search/b3f60365- 847a-4fd4-abdb-747568a1c726 Last accessed 14 July 2023
- MCHW Series NG 5700 – Concrete Repairs see: https://www.standardsforhighways.co.uk/search/55e1b2ae- 296b-42fe-9370-893972ae3a0f Last accessed 14 July 2023
- Christodoulou C, Goodier CI, Austin SA, Glass GK and Webb J, Construction and building materials, 50 (2014), 300-307.
- Bewley D and Stone C, Concrete, 57(5) (June 2023) 43-
- Dodds W, Christodoulou C and Goodier CI, Proceedings of the Institution of Civil Engineers – Construction Materials, 171(4) (2018) 149-160.
- Glass GK, Davison N and Roberts AC, Materials and Corrosion,70(3) (2019) 503-
- Whitmore D, ICRI Webinar: Design Considerations for Galvanic Anodes, 6 December 2022