Authors: Chathumini Samarawickrama, Xiaobo Chen, Paul White, Patrick Keil, Ivan Cole.
This paper was presented at Corrosion & Prevention 2023.
ABSTRACT
The protection of steel and galvanised steel from corrosion is crucial for sustainability, mainly due to the significant carbon footprint associated with the automotive and construction industries, which produce substantial amounts of steel annually. While direct corrosion costs are often considered, the indirect costs resulting from corrosion failures are often overlooked. Therefore, it is imperative to prioritise corrosion mitigation strategies. Protective coatings and corrosion inhibitors are commonly employed to prevent corrosion in steel. However, existing studies primarily focus on using inhibitors in bulk volume electrolytes, neglecting the corrosion protection mechanisms specific to droplet volumes. Droplet corrosion refers to the phenomenon where oxygen depletion at the centre of a droplet, compared to its edges, creates an oxygen gradient within the droplet, leading to corrosion. Since this corrosion mechanism differs significantly from bulk volume corrosion, the effectiveness of corrosion inhibitors may vary accordingly. Various factors, including pH, secondary spreading, relative humidity, salt load density, and others, influence the occurrence of corrosion under droplets. Unfortunately, there is a lack of research on the mechanisms of droplet corrosion and its impact on the efficiency of corrosion inhibitors. To bridge this research gap, this study proposes a rapid electrochemical test method to evaluate the performance of different inhibitors under various conditions specifically related to droplet corrosion. Furthermore, the study delves into a detailed examination of how pH, droplet size, and relative humidity affect the effectiveness of corrosion inhibitors in combating droplet corrosion.
Keywords: steel, droplet corrosion, pH, zinc, corrosion inhibitors
INTRODUCTION
Structural steel naturally undergoes atmospheric corrosion as a result of moisture and oxygen exposure, particularly in coastal areas where the impact can be particularly severe due to the effect of chlorides [1]. Thus, atmospheric corrosion occurs when aerosols and droplets deposit onto metal surfaces, leading to localised electrochemical degradation under a droplet or a thin film. The rate of atmospheric corrosion is highly dependent on the characteristics of the droplets, including their size, shape, pH, salt concentration and many more factors [2-8]. Moreover, it is essential to note that atmospheric corrosion is a complex process influenced by various factors such as temperature changes, moisture condensation in the atmosphere, the hygroscopic effects of surface contaminants, and the subsequent formation of droplets on the metal surface. Previous studies have highlighted the differences in corrosion mechanisms between droplet corrosion and corrosion in conventional bulk volume electrolytes, which is explained by Evan’s droplet theory [2, 7, 9]. In droplet corrosion, corrosion rates are controlled by oxygen diffusion through the electrolyte and measured as diffusion-limited current [6, 7, 10, 11]. Compared to a bulk volume, oxygen is readily available in a droplet as it diffuses through a much shorter path from the atmosphere to the metal surface, thus facilitating corrosion reactions. As per Evan’s theory, a notable phenomenon occurs within the droplet: oxygen diffusion varies across its spatial dimensions. Specifically, in the central region, oxygen encounters a lengthier path to reach the surface, causing it to adopt an anodic role. In contrast, oxygen reaches the surface at the droplet’s edges more rapidly, resulting in a cathodic behaviour. This process creates oxygen and pH gradients within the droplet, which are prominently influenced by the droplet size.
In the context of atmospheric exposure, it is common practice to coat all steel types, except for weathering steel, and to passivate all galvanised steel. In the case of zinc exposed to atmospheric conditions, the goal is to establish a passive film on its surface to inhibit corrosion. Organic corrosion inhibitors have the capability to adhere directly to the metal’s surface and are typically employed for countering corrosion in aqueous environments. Essentially, corrosion inhibitors prevent the formation of such a passive film in applications where passivation is undesirable [12]. While numerous studies have been conducted on corrosion inhibitors using bulk volume electrolytes, there is limited published research on corrosion inhibitors associated with droplet or thin-film electrolytes.
Consequently, there is a scarcity of information regarding the performance of corrosion inhibitors against corrosion under droplets. In recent years, efforts have been made to explore the use of organic and inorganic multifunctional inhibitors to minimise the costs and risks associated with corrosion. However, most of the research in this area focuses on the use of bulk volume electrolytes for electrochemical testing.
Thus, the present study utilises varying droplet volumes through a previously established electrochemical testing methodology, which accommodates both bulk and droplet volumes, to evaluate inhibitor performance. The principal aim is to comprehensively investigate the influence of pH and droplet size on the efficacy of different inhibitors by utilising electrochemical and surface analytical techniques.
METHODS AND MATERIALS
SUBSTRATE PREPARATION
For this study, the chosen substrates are steel and galvanized steel. Two identical metal samples were cut into 52 mm × 20 mm × 1mm size. Cleaned and dried metal samples were then embedded in an epoxy mould (40mm in diameter) with a 10 mm distance in between. Prior to each electrochemical test, exposed faces of the electrodes were abraded using 400, 1200 and 2500 grit SiC papers until a mirror finish was achieved, followed rinsing with ethanol and drying using N2. The samples were stored in a desiccator until use. Both substrates, are of automotive quality supplied by Chemetall GmbH (Frankfurt, Germany).
SOLUTION PREPARATION
The base corrosion medium was a 0.1 M NaCl solution made with de-ionized water. The inhibitor solutions were made to a standard 10-2 M (10 mM) concentration in the chloride base solution. All solutions were stirred using a magnetic stirrer at room temperature until the inhibitors were fully dissolved. The solutions were then aerated with compressed air which ensured that the solutions were oxygen saturated. The pH of the solutions was maintained at a neutral level unless specified otherwise. All used chemicals were analytical grade.
ELECTROCHEMICAL TECHNIQUE
The electrochemical setup and methodology employed in this study closely followed the procedures outlined in the work of C. Samarawickrama et al [13]. This multi-electrode cell system entails the connection of the reference and counter electrode to one metal coupon and the working electrode to another metal coupon, both of which had been previously embedded in an epoxy resin. Subsequently, a polarization test was conducted utilizing a Biologic VMP300 potentiostat to assess the inhibitor’s effectiveness on galvanized steel.
The experimental protocol initiated with an open circuit potential (OCP) scan, which spanned a duration of 10 minutes. Following this initial phase, a polarization test was carried out, involving the application of a potential of -75 mV for a duration of 60 seconds. Subsequently, the system was allowed to attain a steady state once again, maintained at OCP for 30 seconds, and subsequently polarized with an applied potential of +75 mV for an additional 60 seconds. The resultant average current measurements were then employed to compute the inhibition efficiency of the selected inhibitors. It is important to note that a droplet size of 800 µL and a bulk volume of 80 mL were employed in these experiments, which were meticulously repeated three times to ensure precision and reliability. The averaged corrosion currents were subsequently utilized to determine the inhibition efficiency of the inhibitors under investigation.
DROPLET-ON-PLATE TECHNIQUE
An established non-electrochemical technique known as the droplet-on-plate method, as originally introduced by N. Azmat et al [6, 14] has been employed in prior studies to assess corrosion under a droplet. This method involves evaluating the mass loss that occurs over a specified time period. Additionally, a rapid screening technique has been employed in which a series of droplets, each containing various inhibitors, are placed on the substrate surface to quantify the extent of corrosion and distinguish between effective and ineffective inhibitors. In this study, a similar approach has been used but to monitor the pH distribution within a droplet in the absence and presence of a selected number of inhibitors at different time intervals.
Preceding the placement of the droplets onto a steel plate, a universal indicator was introduced into the solutions. Three separate 100 µL droplets, each containing solutions with pH values of 3, 7 and 10 were carefully positioned adjacent to each other on the substrate surface. Images were captured at various time intervals, continually documented until the droplets had completely evaporated. This experiment was conducted under ambient humidity conditions and at a standard temperature. A macrophotography setup was employed for image acquisition, and the resulting images were subjected to post-processing to enhance colour saturation for subsequent analysis.
RESULTS
ELECTROCHEMICAL TESTING
The established rapid screening technique was employed to assess the inhibition efficiencies of a variety of inhibitors on galvanized steel, all maintained at a concentration of 10 mM, across different initial pH values, specifically pH 3, 7, and 10. These evaluations were executed in both bulk and droplet volumes, and the outcomes are presented graphically in figure 1 below.
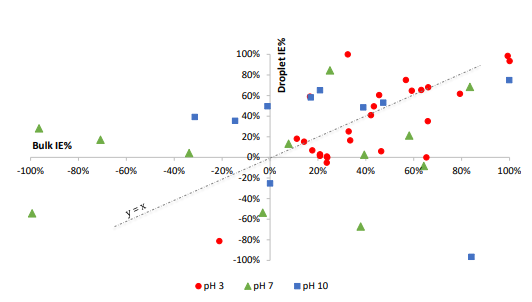
FIGURE 1: COMPARISON OF INHIBITION EFFICIENCIES IN BULK AND DROPLET VOLUMES AT PH 3, 7 AND 10 ON GALVANIZED STEEL
Based on the collected data, it is evident that inhibitor solutions initialized at a starting pH of 3 exhibit a consistent linear trend, implying comparable performance in both bulk and droplet volumes. Notably, the majority of the selected inhibitors demonstrate notably favourable performance within this pH range when compared to other initial pH values. Interestingly, at pH 7, inhibitors manifest a distinctly scattered pattern, with a greater number of inhibitors exhibiting outlier behaviour. This suggests that with effective inhibitors, a majority of them exhibit moderate to high inhibition in bulk volumes and low to moderate inhibition in droplet volumes.
In Figure 1, it is apparent that only a subset of inhibitors at pH 10 is visible, as others yield negative inhibition efficiencies that extend beyond the axis limits, effectively functioning as corrosion promoters. However, many of the tested compounds cluster around a 50% inhibition efficiency in droplet environments, displaying varying positive and negative percentage inhibition in bulk solutions. Thus, it can be inferred that pH 10 conditions tend to favour corrosion inhibition within droplets to a relatively greater extent.
To provide a more detailed examination of these differences, Figure 2 presents the results for an inhibited solution featuring 10 mM 5-methyl-1,3,4-thiadiazole-2-thiol at various pH levels in both bulk and droplet volumes. This plot serves as a comparative analysis of inhibition efficiencies, with the polarization test conducted after 10 minutes of Open Circuit Potential (OCP) measurements.
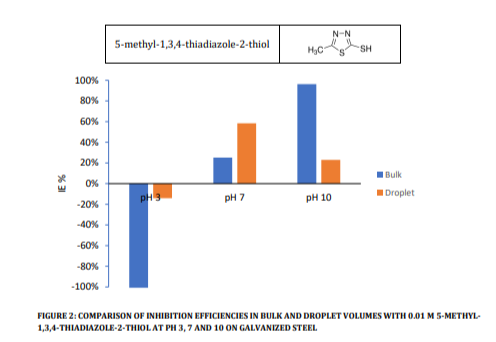
Figure 2 displays the presence of significant discrepancies in inhibition efficiencies between bulk and droplet volumes for this inhibitor on galvanized steel substrate. Under acidic conditions, both scenarios exhibit poor inhibition and accelerated corrosion within bulk solutions. Conversely, under neutral conditions, the inhibitor demonstrates superior performance within droplets, while the alkaline droplet results in comparatively diminished effectiveness. It is noteworthy that at pH 10, corrosion currents measured for both inhibited and uninhibited conditions are notably lower than those observed at pH 3 and 7, a phenomenon potentially attributable to oxide formation.
DROPLET-ON-PLATE TECHNIQUE
A comprehensive series of experiments were systematically conducted employing the droplet-on-plate test methodology. The primary objective of these experiments was to meticulously monitor the dynamic pH gradients and their evolution within the droplets until the point of complete evaporation. To facilitate comparative analyses, both inhibited and uninhibited solutions, featuring droplets of identical dimensions, were placed on a cleaned steel plate.
Notably, within the droplet, an apparent transition occurs, wherein the central region gradually assumes a yellow hue, indicative of an increasingly acidic nature. In contrast, the encompassing area exhibits a notable bluish tint, signifying an alkaline character. It is crucial to be cautious in interpreting these colour changes over time, as the ongoing corrosion of the substrate itself introduces additional complexities. Specifically, the formation of iron corrosion products on the surface contributes a yellow tint to the visual observations, potentially misinterpreting the assessment of pH-induced colour variations.

At pH 7, approximately 10 minutes into the experiment, there is a notable transition in colour to a deeper purple hue. This shift in coloration is indicative of an increase in alkalinity, attributed to the heightened generation of OHions at the periphery of the droplet. Conversely, the droplet subjected to a pH of 10 for 25 minutes exhibits regions characterized by a red hue, potentially signifying the presence of anodic sites. A noteworthy contrast is observed in the pH 3 droplet, where a substantial degree of corrosion is evident primarily at the droplet’s edge, with comparatively less corrosion occurring at its centre. Over time, as the droplet undergoes spreading, the outer region beyond the corroded circumference appears to exhibit an increased alkaline character, suggestive of cathodic behaviour. While significant spreading is observed in the other two droplets, there is an absence of significant corrosion in the cathodic region.
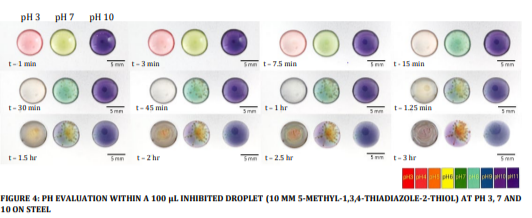
The same experimental procedure was conducted using a 10 mM concentration of 5-methyl-1,3,4-thiadiazole-2-thiol at various pH ranges, and the resulting outcomes are presented in Figure 4.
FIGURE 4: PH EVALUATION WITHIN A 100 µL INHIBITED DROPLET (10 MM 5-METHYL-1,3,4-THIADIAZOLE-2-THIOL) AT PH 3, 7 AND 10 ON STEEL
It is essential to emphasize that, under ambient conditions, the uninhibited NaCl droplet exhibited complete evaporation within a relatively short span of 2 hours. In contrast, the inhibited droplet, regardless of the pH values tested, exhibited a substantially prolonged evaporation time, exceeding 4 hours. However, this experiment can be conducted in a humidity chamber with controlled humidity and temperature to investigate the effects of wetness over a prolonged period of time. It is noteworthy that, contrary to the observations in Figure 3, the applicability of Evan’s theory is not evident in this context, as all three droplets exhibit distinct behaviours. However, a notable commonality is observed in the alkalinity trend, where both the acidic and neutral droplets undergo a transition towards increased alkalinity.
While Figure 3 demonstrates a uniform corrosion pattern across the droplets, Figure 4 portrays distinct signs of pitting corrosion within the neutral droplet. In contrast, the acidified droplet maintains a uniform corrosion pattern, and the alkaline droplet exhibits minimal to negligible corrosion until the 3-hour mark. The acidified droplet is the sole instance where secondary spreading around the droplet perimeter is observed, a phenomenon absent in the other droplets. With extended exposure, the highly alkaline droplet undergoes a perceptible colour change to a dark blue hue, indicative of a decline in pH levels.
DISCUSSION
Corrosion protection is commonly achieved through protective coatings and/or corrosion inhibitors. In the case of active protection by inhibitors, due to scratches or damages on the coatings, inhibitor within these coatings is released into the solution to shield the exposed metal and provide protection. Therefore, these leached inhibitors need to be active in the solution. This rationale underlies the conduct of electrochemical testing with inhibitors in solution rather than on pre-coated metal substrates, as it closely simulates real-world conditions and responses.
Droplet corrosion is profoundly influenced by the presence of pH gradients within the confined microenvironments of the droplets. It is important to gain insight into how these pH variations might critically influence the efficacy of corrosion inhibitors. In contrast to bulk volume scenarios, where pH alterations occur upon exposure, their impact is considerably less due to the diffusion processes that operate within the larger solution volumes. The dynamic and ever-evolving local conditions within a droplet environment can cause the formation of distinct oxide species at spatially different locations. Over time, a complex interplay of factors, including hydration reactions at the droplet centre and oxygen reduction at its periphery, leads to the formation of pH gradients.
Electrochemical assessments conducted on galvanized steel revealed a noteworthy increase in corrosion inhibition within acidified droplets. This enhancement can be attributed to the dissolution of pre-existing oxide layers in highly acidic environments, facilitating direct interactions between inhibitor molecules and the metal surface. At pH 7, most inhibitor droplets perform differently to bulk volume and the effective inhibitors at high alkalinity, appear to perform significantly better at droplet volume than bulk volume. The Pourbaix diagram at pH 10 outlines the formation of a passive zinc film, with the precipitated layer potentially serving as an effective anodic barrier. In the case of droplets, the increased diffusion of oxygen to the metal surface, coupled with the presence of a thin and porous precipitate layer, can accelerate the rate of metal dissolution, leading to pronounced corrosion. However, a thicker oxide layer forms over extended exposure durations, creating a more robust protective shield [15].
Figure 2 provides compelling evidence of substantial disparities in corrosion inhibition between bulk and droplet volumes, especially when steel is employed as the substrate. This disparity underlines the potential for variation in inhibitor performance within the context of droplet corrosion. It is worth noting that the inhibitor 5-methyl1,3,4-thiadiazole-2-thiol is recognized for its effectiveness in bulk volumes [16]. The data presented in this study were obtained after a 10-minute interval, which may explain the observed poor inhibition efficiency at neutral pH. This phenomenon may be attributed to the inhibitor’s film-forming nature, necessitating a longer time to display its optimum capability.
Figure 3 illustrates the pH dynamics within an uninhibited droplet, while Figure 4 depicts a droplet subjected to inhibition. In accordance with Evan’s theory, the droplet’s geometry gives rise to the formation of localized cathodic and anodic regions. The shortest path for oxygen transport is presumed to occur along the droplet’s periphery, presenting the circumference as the cathode and the centre of the anode. This phenomenon is visually validated by the series of images presented in Figure 3. Neutral and alkaline droplets exhibit the anticipated pronounced pH gradients characteristic of droplet corrosion. However, in the case of the inhibitor-containing droplet, these pH gradients are less pronounced during the initial 2-hour period. Remarkably, the overall pH of the neutral droplet becomes progressively more alkaline, suggesting a markedly higher rate of hydroxyl ion formation compared to metal dissolution, thereby elevating the overall pH. Once sufficient pitting corrosion has taken place, a pH gradient has formed with the edge being more alkaline and the areas surrounding the pits becoming more anodic. It is noteworthy that only the acidified droplet displays secondary spreading as secondary spreading tends to be more prominent with higher corrosion rates. In line with Evan’s theory, the centre is assumed to function as the anode, while the secondary spread region may assume a cathodic role, with the entire area beneath the droplet serving as the anode. This configuration arises from the significantly reduced path of oxygen diffusion to the metal surface within the secondary spread area. However, in Figure 4, with the onset of rust formation, the droplets begin to spread out, possibly attributable to changes in wettability, a phenomenon absent in the inhibitor-containing droplet.
The significance of investigating pH effects becomes apparent when considering these findings, which reveal variations in inhibitor performance between bulk and droplet volumes. Thus, comprehending the impact of pH is crucial among the numerous factors associated with droplet corrosion and inhibitor efficacy.
CONCLUSIONS
The primary objective of this investigation was to assess the influence of pH on corrosion in both droplet and bulk volumes for both steel and galvanized steel, in the presence and absence of corrosion inhibitors. Rapid screening electrochemical tests were employed to discern trends across various initial pH levels. At a pH of 3, a consistent linear trend emerged, indicating that the majority of inhibitors exhibited similar performance in both bulk and droplet volumes on galvanized steel. This phenomenon can be attributed to the high acidity at pH 3, which dissolves any existing oxide layers on the metal surface, enabling inhibitors to directly engage with the metal. Conversely, at pH 7, notable disparities in inhibitor performance were observed, with a greater number of inhibitors proving to be more effective in the bulk volume compared to the droplet volume. In contrast, at highly alkaline pH levels, the inhibitors demonstrated enhanced efficacy in the droplet volume. The pH evolution, as monitored through the droplet-on-plate test, offered clear evidence of pH gradient formation within the droplets. As anticipated, uninhibited droplets exhibited more rapid gradient development compared to their inhibitor-containing counterparts. Acidified droplets exhibited a unique characteristic in that they induced secondary spreading, although it is noteworthy that all droplets gradually expanded over time. This expansion could potentially be attributed to the accumulation of rust within the droplets, which altered the solution density. The central focus of this investigation is to examine the impact of pH on droplet corrosion and its consequential effects on inhibitor performance. It is worth emphasizing that pH exerts a notably greater influence on droplet corrosion compared to corrosion in bulk volumes.
References
[1] L. Di Sarno, A. Majidian, and G. Karagiannakis, “The Effect of Atmospheric Corrosion on Steel Structures: A State-of-the-Art and Case-Study,” Buildings (Basel), vol. 11, no. 12, p. 571, 2021.
[2] I. Cole, N. Azmat, A. Kanta, and M. Venkatraman, “What really controls the atmospheric corrosion of zinc? Effect of marine aerosols on atmospheric corrosion of zinc,” International materials reviews, vol. 54, no. 3, pp. 117-133, 2009.
[3] R. Lindström, J.-E. Svensson, and L.-G. Johansson, “The Influence of Salt Deposits on the Atmospheric Corrosion of Zinc. The Important Role of the Sodium Ion,” Journal of The Electrochemical Society, vol. 149, pp. B57-B64, 02/01 2002.
[4] I. S. Cole, T. H. Muster, S. A. Furman, N. Wright, and A. Bradbury, “Products Formed during the Interaction of Seawater Droplets with Zinc Surfaces: I. Results from 1- and 2.5-Day Exposures,” Journal of the Electrochemical Society, vol. 155, no. 5, p. C244, 2008.
[5] I. Cole, T. Muster, D. Lau, N. Wright, and N. S. Azmat, “Products formed during the interaction of seawater droplets with zinc surfaces: II. Results from short exposures,” Journal of the Electrochemical Society, vol. 157, no. 6, p. C213, 2010.
[6] N. S. Azmat, K. D. Ralston, B. C. Muddle, and I. S. Cole, “Corrosion of Zn under acidified marine droplets,” Corrosion Science, vol. 53, no. 4, pp. 1604-1615, 2011/04/01/ 2011. http://www.sciencedirect.com/science/article/pii/S0010938X11000667
[7] T. H. Muster et al., “The atmospheric corrosion of zinc: The effects of salt concentration, droplet size and droplet shape,” Electrochimica acta, vol. 56, no. 4, pp. 1866-1873, 2011.
[8] L. Guo et al., “The effect of relative humidity change on atmospheric pitting corrosion of stainless steel 304L,” Corrosion Science, vol. 150, pp. 110-120, 2019/04/15/ 2019. https://www.sciencedirect.com/science/article/pii/S0010938X18317426
[9] S. C. Morton and G. S. Frankel, “Atmospheric pitting corrosion of AA7075-T6 under evaporating droplets with and without inhibitors,” Materials and Corrosion, https://doi.org/10.1002/maco.201307363 vol. 65, no. 4, pp. 351-361, 2014/04/01 2014. https://doi.org/10.1002/maco.201307363
[10] I. S. Cole, N. S. Azmat, A. Kanta, and M. Venkatraman, “What really controls the atmospheric corrosion of zinc? Effect of marine aerosols on atmospheric corrosion of zinc,” International materials reviews, vol. 54, no. 3, pp. 117-133, 2009.
[11] I. S. Cole, “Recent progress and required developments in atmospheric corrosion of galvanised steel and zinc,” Materials, vol. 10, no. 11, p. 1288, 2017.
[12] X. G. Zhang, Corrosion and electrochemistry of zinc. Springer Science & Business Media, 1996.
[13] S. P. C. Samarawickrama, Xiao-Bo Chen, Rou Jun Toh, P. Keil, I. Cole, “Comparison betwen the performance of a corrosion inhibitor by droplet and bulk volume for AA6014,” in Corrosion and Prevention, Newcastle, Australia, 2021: Australasian Corrosion Association.
[14] N. S. Azmat, K. D. Ralston, B. C. Muddle, and I. S. Cole, “Corrosion of Zn under fine size aerosols and droplets using inkjet printer deposition and optical profilometry quantification,” Corrosion Science, vol. 53, no. 11, pp. 3534-3541, 2011/11/01/ 2011. http://www.sciencedirect.com/science/article/pii/S0010938X11003465
[15] S. Thomas, I. S. Cole, M. Sridhar, and N. Birbilis, “Revisiting zinc passivation in alkaline solutions,” Electrochimica Acta, vol. 97, pp. 192-201, 2013/05/01/ 2013. https://www.sciencedirect.com/science/article/pii/S0013468613004040
[16] Q. H. Zhang, B. S. Hou, and G. A. Zhang, “Inhibitive and adsorption behavior of thiadiazole derivatives on carbon steel corrosion in CO2-saturated oilfield produced water: Effect of substituent group on efficiency,” Journal of Colloid and Interface Science, vol. 572, pp. 91-106, 2020/07/15/ 2020. https://www.sciencedirect.com/science/article/pii/S002197972030357X
AUTHOR DETAILS
Chathumini Samarawickrama is a PhD student under the School of Mechanical & Manufacturing Engineering at RMIT University on collaboration with BASF Coatings, Germany. She’s currently working on the discovery of inhibitors with the main focus on droplets. Her research interests are focused on corrosion protection and development of corrosion inhibitors.
Dr. Xiaobo Chen is Senior Lecturer at School of Engineering, RMIT University. His research expertise is multidisciplinary and spans from chemistry and materials science through to corrosion, electrochemistry and biomaterials. Over the last decade, his endeavours have been aiming to provide functional characteristics upon surface of light metals to meet the requirements for a large range of engineering applications in automotive, 3C and biomedical industries.
Dr Paul White is a Principal Research Fellow at RMIT University. He has developed new high-throughput techniques for corrosion inhibition together with the data capture and data analysis of the resulting cavalcade of ensuing experimental data, resulting in various patents relating to corrosion inhibitor protection of aerospace aluminium alloys and steel.
Dr Patrick Keil is Research Fellow at the Coatings division of BASF. He has a long track of experience in corrosion and surface science related research within academia and industry. His research interests are focused on development of new protection technologies, to understand the relationship between corrosion and resulting material performance and the development of new methodologies to assess corrosion.
Prof Ivan Cole is a Professor of Engineering at RMIT. Research interests are corrosion modelling and sensing and development of new inhibitors. His modelling work focuses on linking scales from the molecular to the continental to understand both the fine scale process and the factors controlling corrosion. His sensor work concentrates on AI to enhance sensor data interpretation while he is developing rapid discovery methods (for inhibitors) combining molecular modelling, AI and robotic electrochemistry.