Author: Ian D. MacLeod
This paper was presented at Corrosion & Prevention 2023
ABSTRACT
Rock art images in Western Australia are found from the Kimberley region in our monsoonal north through the arid and rocky Pilbara to the semi-arid lands of the middle latitudes. Images take on many forms from pigment painted rock surfaces to engraved rocks where the images have been carved through the external rock patina. As weathering of the exposed parent rock proceeds, the tonal contrast between the weathered patina and the substrate diminishes. Natural weathering from rain, and chemical reactions with plant and microbial metabolites, alter the pigments leading to loss of cultural information. Decay mechanisms have been discerned through a combination of microclimate modelling, applied mineralogy and in-situ studies of pH, chlorinity and redox voltages. This information enables the development of effective management strategies for heritage preservation.
Keywords: rock art, climate, weathering, mineralogical changes, corrosion mechanisms
INTRODUCTION
Australia has one of the richest collections of indigenous rock art in the world. It is a mixture of painted and engraved images formed by the application of pigments of various hues and engraving through rock surface patinas. The state of Western Australia has been occupied by Aboriginal people for more than 55,000 years however since the arrival of white settlers in 1829 at the Swan River Colony many areas over the 2.6 million square kilometres have undergone significant cultural upheaval resulting in the loss of connection with country and the falling away of traditions like the periodic repainting of Wandjina images which ensured the continuity of religious practices and associated rock art [1]. Chemical reactions involving the pigments change calcareous pigments into different minerals which can at times be more stable and resistant to localised weathering reactions. The availability of white clays such as kaolinite from weathered granites provides a different tonal palette than carbonate-based materials but they are notoriously sensitive to moisture both directly through hydration changes and corrosion involving biological activity. Rock art, unlike traditional corrosion, does not involve parent metal being subject decay but is associated with changes in oxidation states of key elements in the rock patinas. Western Australia extends from the monsoonal north Kimberley through the arid and rocky Pilbara through the Wheatbelt of arable and semi-arid lands of the middle latitudes to calcareous cave sites in the south-west of the state.
Owing to the very large distances involved in field work and the logistical costs in-situ inspections, regular visits to sites are prohibitive for community-based organisations. A combination of the application and empirical validation of microclimate models involving heat exchange processes resulting from air movement and radiative heat loss to the open sky has allowed microclimate predictions within and across sites to be made. This approach helps to identify the risk of decay mechanisms leading to the development long-term preservation strategies to preserve and promote indigenous management of the state’s rock art heritage values.
METHODS AND MATERIALS
The pH data was recorded using a flat surface pH electrode (VWR International LLC model no. W7567287) connected to a TPS Aqua pH/ORP/°C meter; originally the TPS business was a Transistor Power Supply company but now they are a Brisbane-based manufacturer and supplier of water quality testing equipment. The pH electrode was calibrated daily using pH 4 & 7 buffers. Readings were taken by instilling two drops of distilled water between the rock surface and the electrode after which stable values were obtained within 30–60 seconds. Chloride ion activity was measured using a TPS WP-90 ion-pH-mV-°C meter connected to an Orion Thermo 1609-186881 chloride ion-specific electrode calibrated daily at 1,000 and 100 ppm. In this case two drops of a 0.05 M sodium nitrate electrolyte solution were introduced to the edge of the interface between the electrode and readings stabilised within 60 seconds. Redox measurements were taken using a 2 mm o.d. platinum wire electrode pushed through a sponge rubber mat wetted with local tap water and an Ag/AgCl (3M KCl) reference electrode was held on the wetted rock at the edge of the sponge against the platinum electrode. The reference electrode was calibrated using a saturated quinhydrone solution at pH 4.0 and had a voltage of + 0.210 volts versus the standard hydrogen electrode [2].
Rock surface washings using distilled water were collected using an inert rubber ring attached to a short length of 12 cm diameter PVC sewer pipe which acted like a dam and the liquid was transferred to inert laboratory storage bottles using disposable syringes. The liquids were initially kept in an insulated bag cooled with freezer bricks and later stored in a domestic refrigerator until returned to Perth for analysis by the Chemistry Centre of Western Australia. Adjacent surfaces were used for the collection of anion washings as the cation and anion analyses were determined using different laboratory procedures. Cation analyses were made using Inductively Coupled Plasma[1]Mass Spectrometry and the anions determined using ion chromatography. The most abundant soluble materials were anions and cations associated with sea water, namely Na+, K+, Mg2+, Ca2+, Cl- and SO42-. Their concentrations varied across the Burrup and were determined by the microenvironments created according to prevailing winds, proximity to the sea, and occasional rainfall including deluges from cyclones. Cations associated with weathered igneous and metamorphic rocks were detected at the ppm to ppb ranges. The microenvironment of the rocks, assessed through the washings, showed good agreement (± 5%) within each site location.
RESULTS
REACTIONS OF BIRD GUANO WITH CALCITIC AND KAOLINITIC PIGMENTS
Nearly four decades ago a series of reports from rock art conservation teams [3,4] indicated that unexpected preservation of readily weathered pigments like calcite and kaolinite may occur in arid environments when they interact with aqueous extracts of avian guano. A project to systematically assess the operation of the phenomenon on paintings at Walga Rock was funded by the Australian Institute for Aboriginal and Torres Strait Islander Studies (AIATSIS). Walga Rock is a granite monolith some 1.5 km long and up to 0.5 km wide located 45 km southwest of Cue in the Murchison district of Western Australia (Figure 1) and the paintings are on southwestern side of the rock. Prior to commencing experimental laboratory studies using fresh pigments and aged zoo-sourced guano extracts, microclimate assessment concluded that fog and driving rain, rather than dew, were the most significant sources of water [5]. The site is in a semi-arid region and the nearby town of Cue has a highly variable annual rainfall averaging 224 mm within 42 rain days, most occurring in the autumn and winter months. There are also occasional remnants of tropical cyclones through the region. The average maxima vary from 38oC in January to 18.4oC in June with average minima of 23.3oC in January to 7.0oC in June [6]. The field component of the AIATSIS project, designed to examine the interactions of pigments and water borne minerals found in avian guano, unexpectedly demonstrated the degree of preservation was inversely proportional to the distance from where the avian guano was deposited beneath perches high on the granite monolith (Figure 2). The reactions of kaolinitic and calcitic pigments with urea, proteinaceous materials, and phosphorus-containing compounds were studied by x-ray diffraction (XRD) and conventional wet chemical analyses. Some albumen proteins have been shown to survive prolonged exposure to desiccation and high levels of ultra-violet radiation [7].

Pigment samples were collected and identified using XRD to test their reactions with guano extracts; kaolinite from nearby Mt Magnet, while calcite came from local mineral deposits. Surface acidity measurements were taken both inside and outside the vertical flow lines over three main areas. The unaffected granite surface pH was 5.9 <pH granite> 5.3 and within the guano flow area a narrower and more acidic range of 5.1<pH guano flow >4.9 was measured. The pH of areas outside the avian guano showed less variability with height. Small samples of the rock substrate from within both zones were taken following pH measurement. A mixture of fresh swallow, kestrel, and owl guano obtained from the Perth Zoo had a mean pH of 6.4±0.1 which after two months drying in the conservation laboratory dropped to pH of 5.8±0.3. Continuing maturation and biological activity in the zoo poo resulted in a final pH of 5.4, which was the same as guano collected at Walga Rock. A wide range of phosphate minerals were found in the bird guano samples collected from the zoo and onsite and the presence of pyrophosphates in both aged samples is consistent with dehydration and cation exchange reactions under the hot and acidic surface conditions on the rock. Fresh droppings contained aragonite which had been biologically converted to whewellite, CaC2O4.H2O, in the aged guano. There is clear evidence in the sequence of the paintings that overpainting had been practised extensively at the site and, despite the inherent physical instability of calcite pigments, it is likely the images predated European contact. Nevertheless, it is possible that the observed chemical transformations occurred in a relatively short time span. Surface analysis by back scattered SEM secondary imaging showed pigments from the “preserved” surfaces had well developed grains consistent with dissolution/re-precipitation.
The presence of rustumite, Ca10(Si2O7)2(SiO4)(OH)2Cl2, indicated that kaolinite, Al2Si2O5(OH) had interacted with soluble Ca2+ from the fresh guano deposits and in doing so the chemical “corrosion” resulted in a product with improved adhesive and cohesive properties. The reaction of kaolinite with aged guano resulted in no new silicate minerals. Frequency shifts in the FTIR absorption spectra of the residual mucoproteins in aged guano indicate interactions with kaolinite. Given that the old guano contained 4.2 wt% phosphorus, it was not unexpected to find phosphate minerals among the reaction products viz., Na2CaMg(PO4)2, CaMgP2O7 and Ca3Mg3(PO4)4 and their formation is consistent with the original pigments undergoing a range of ion-exchange processes as the guano-containing water flowed over them. Lattice substitution of magnesium for calcium commonly occurs in the formation of mixed carbonate and phosphate deposits. A possible reaction scheme for the pyrophosphate mineralisation involves a substitution and a dehydration step in which the highly soluble bicarbonate ion is washed away:
2 CaCO3 + 2 H2PO4- → Ca2P2O7 + H2O + 2 HCO3- (1)
The identification of the fluoride-substituted phosphates and the range of pyrophosphates/phosphates containing mixtures of alkaline earth cations (Mg2+, Ca2+, Ba2+) in the interaction zones of the guano flow strongly support water[1]borne phosphates reacting the weathered rock surfaces as well as the pigments. Like metallic corrosion, the major mechanism for guano phosphate transport is likely associated with real surface wetness. The low frequency of rain or fog suggests that the conversion of calcite to apatite-like phosphate minerals would be overall a very slow process, with individual reactions under optimal conditions occurring relatively rapidly. Apart from the formation of rustumite from kaolinite the presence of pyrophyllite, Al2Si4O10(OH)2 on the rock surface and greenalite, Fe3Si2O5(OH)4, from reaction with fresh avian guano provides an insight into the possible binding mechanism between the altered kaolinite and the weathered granite surfaces. The substitution of cations in the silicate sheet layer lattices alters the fundamental hydration properties of the pigments rendering the inherently unstable high surface area kaolinitic materials less subject to the stresses of alternating cycles of rehydration and dehydration [8].
Corrosion of Kimberley rock art pigments:
carbonates into oxalates. The Kimberley region in Western Australia is recognised for the wealth of painted Wandjina images in which the vivid white faces are decorated with eyes, ears, nose but with no mouth. Once the creative energy associated with naming of people and the environment had finished, Wandjina had no need for a voice, for their work was done. Wandjina are the very powerful creative spirit beings who bring the annual monsoonal rain to the Kimberley. The white pigment is composed of the local mineral huntite, Mg3Ca(CO3)4 a mineral left over from drying of ancient sea beds. It is further processed by levigation in water to a particle size which imparts its characteristically brilliant whiteness and when mixed with water results in a suitably viscous suspension for application without binders. The renowned pigment was widely traded however, its physical properties and method of application predispose it towards serious deterioration [9]. Its use has been maintained in more modern portable paintings by, for example, the late Kimberley artist Rover Thomas, where its structural instability in the face of RH change continues to cause problems even in relatively well controlled environments [10]. In rock art it is also used as a background to other coloured minerals covering the full visual palette for zoomorphic images and stylised representations of natural phenomena such as lightning. Field work in the Kimberley region of Western Australia in 1990 and 1992 provided the opportunity to examine pigment samples from Aboriginal rock art sites in the Napier Ranges and on the Mitchell Plateau of Western Australia in both the wet and the dry seasons (Figure 3 & 4).
During this work mineralogical analysis showed that many of the Wandjina image pigments contained quite large proportions of whewellite and since there are no deposits of this much less environmentally reactive mineral suitable for pigments in the region it appeared to be derived from in situ chemical transmutation of huntite. Mineral phases were identified by x-ray diffraction (XRD), microscopic examination using environmental SEM, and traditional wet chemical analyses. The microclimates of the rock art shelters within which the paintings were made were also recorded and correlated with results from local meteorological weather stations. The distinct white, cream, and grey pigment layers included a variety of minerals in addition to huntite and whewellite, and the chemical processes involved in their conversion into other mineral forms less prone to spalling in the wet season were assessed. The underlying microclimatic differences within and between Napier Range (Devonian limestone) and Mitchell Plateau (siliceous sandstone) painting sites were found to be key determinants in the rate of conversion and corrosion reactions.
Identification methods
Pigment sampling was carried out under the supervision of and in accordance with the views of traditional owners, which included avoiding visible signs of intervention. Already loose pigment flakes were removed and examined under visible and ultraviolet light at 100-300 magnification using dark field incident illumination. A portion of the sample was lightly crushed to break up aggregates to estimate particle sizes before a small portion was dispersed in a drop of methanol to separate the pigment particles and to examine them for the presence of organic binding material, of which there were no traces. This is consistent with traditional accounts of painting techniques which include the application of a water-based dispersion using brushes and spraying from the mouth [11]. The most common phase at 27.5% was huntite and its conversion or corrosion product whewellite, CaC2O4.H2O, was present in similar proportion. The next most common mineral was calcite, CaCO3, at 23%; twice as common as the grey, white mineral dolomite, MgCa(CO3)2. Almost as common as the dolomite was the hydrated calcium sulphate gypsum. Red hued minerals such as hematite (Fe2O3) and goethite (α-FeO.OH) accounted for 5% of the identified phases and the white siliceous minerals illite, (K,H30)Al2Si3AlO10(OH)2, and antigorite, Mg3Si2O5(OH)4, (white to green blue) were present in similar proportion. There were only two examples of the calcium oxalate dihydrate weddellite, CaC2O4.2H2O, both of which occurred as botryoidal deposits near driplines in the siliceous sandstone sites in the Mitchell Plateau and are considered to be an alteration product rather than a pigment. The list of identified phases and their frequencies are listed in Table 1.
Mounted and polished cross-sections were used to visualise successive mineral accretions – most likely calcite, gypsum, or oxalates. Electron microscopy revealed characteristic rhombohedral platelike huntite crystals of a uniform 1-2 μm particle size. In some cases, the sections showed that the whewellite consisted of many discrete layers, consistent with its formation during seasonal variations in biological activity. The oxalate anion is formed by epilithic and endolithic cyanobacteria and lichens as well as by anthropogenic air pollution. In the remote Kimberley region air pollution can be discounted as a source of oxalate [13, 14]. The varying colours of the deposits are also partly a result of the incorporation of charcoal from burnt spinifex grasses. Although formation of oxalate crusts at rock art sites in Australia (and elsewhere) is well-known [10],the lack of cultural or analytic information suggesting their use as pigments supports the supposition that whewellite and its higher hydrate weddellite are pigment alteration products.
In the Kimberley, it is very likely that plant metabolites are the principal source of oxalate ions. Microbial studies from pigment samples collected in the wet and dry seasons show significant microbiological activity [15]. In a different cultural context, the formation of whewellite (CaC2O4.H2O) and weddellite (CaC2O4.2H2O) through displacement reactions on the surface of gypsum crystals on marble has been investigated by del Monte and Sabbioni [13]. For the Kimberley and Mitchell Plateau samples, the transformation mechanism was confirmed by reacting huntite with slightly acidic solutions (5.9 <pH>5.5) containing oxalate anions for 10 days. The monohydrate oxalate was preferentially formed at slightly higher than room temperatures, while the dihydrate weddellite predominantly crystallises at lower temperatures. There was significant preferential mobilisation or corrosion of magnesium as the aqueous filtrate contained 16 times the stochiometric magnesium content. Other minerals had also been formed with XRD identifying whewellite, brucite (Mg(OH)2), dolomite (MgCa(CO3)2) and vaterite syn. (CaCO3), which strongly supports a series of equilibria (equations 2-4) involving mobilisation of calcium from the huntite and its precipitation as whewellite. Rock art site temperatures of 32 ± 10oC – ten degrees above the laboratory values – would assist in driving the reaction (equation 2) towards completion. The stepwise equations explain the mineral changes in the whewellite formation experiment,
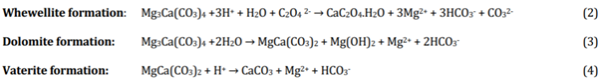
Independent sampling confirmed that dolomite was present via corrosion of huntite and not as a pigment. If the image painters had used a similar source of huntite pigment, then the dolomite had probably come from the thermodynamically favoured route for the dissolution of huntite to produce dolomite and magnesite (MgCO3). The presence of the intermediate conversion mineral of dolomite in the pigments identified in Table 1 is good evidence that the conversion process is the most probable cause of this partly leached form of huntite being observed as a pigment on the rock art on sites in the Napier Range. It is possible that some of the calcite observed as a pigment may have been derived from dissolution of dolomite into calcite and magnesite or some other form of magnesium. The presence of dolomite and calcite as pigments in the Kakadu sites (Northern Territory) may reflect original huntite pigments having been partially or totally converted into the calcium-rich forms by reacting with oxalate and sulphate free acidic rainwater [3].
The influence of short-range microenvironmental differences are evidenced by one example where a separation of only one metre resulted in predominant pigment transformation to whewellite in one location and calcite in the other. Bacterial counts correlated with oxalate formation, can differ by more than two orders of magnitude within the distance of a few metres [15].
Many images had suffered weathering with the loss of large amounts of huntite, however on some sites the conversion of huntite into whewellite had resulted in the preservation of whole images. From SEM images it was determined that the huntite samples had a surface area of 13.5 square metres per gram, and water adsorption studies showed that the plate-like pigments absorb multilayers of water. The first monolayer is complete at 60% RH and thereafter the pigment acquires multiple layers of water altering its volume and making it very prone to physical displacement and chemical corrosion reactions (Figure 5). The presence of acidic rain during the wet season delivers liquids containing sulphate and oxalate resulting in the formation of calcite, gypsum (CaSO4.2H2O) and whewellite. Because the nitrates of calcium and magnesium are soluble, no nitrate containing minerals form during the thunderstorms of the wet season. Calcite and oxalate pigment films are difficult to differentiate under the light microscope as they have similar inclusions and structures. Chemically, the whewellite samples contain a consistently higher concentration of sulphur, which probably reflects the presence of sulphates along with the oxalate produced during the wet seasons.
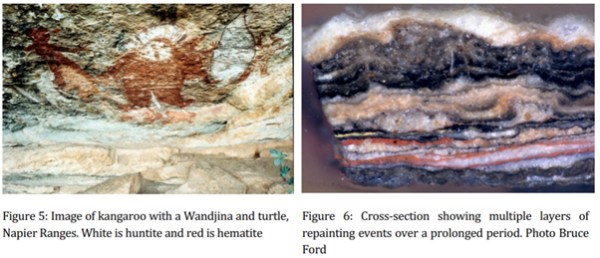
Wet season measurements in 1992 showed that significant conversion of huntite into dolomite had already taken place within five years of a cultural repainting at a Kimberley site. Hydration/dehydration cycling by the direct impact of sunlight on painted surfaces explains the rapid deterioration of freshly repainted huntite particularly during the wet season. Another sample of the recently painted surface showed that in the absence of oxalate and other anions huntite had been converted to dolomite (Equation 3). The black deposits in Figure 6 are from spinifex charcoal but no dating was undertaken at the request of the traditional owners who considered it irrelevant to the cultural and religious significance of the images.
Corrosion of weathered gabbro and granophyre rocks in the Pilbara
“Corrosion” of rock surfaces relates to the processes associated with dissolution of metal ions from the weathering crusts overlying the parent rocks into which the Pilbara engravings were incised. The area is known as Murujuga, meaning ‘hip bone sticking out’ in the local Aboriginal language, and has a uniquely high density of engraved rocks with more than 1,100 individual motifs per square kilometre [16]. In the arid zone of Murujuga, rainfall is dominated by periodic massive rainfall events from cyclonic storms crossing the coast and bringing up to 160 mm of rain in a day, which is just over 50% of the annual mean 313 ± 58 mm. Rainfall from thunderstorms is naturally acidic which makes differentiation of natural and anthropogenic nitrate sources fraught with difficulty. The reason why the monitoring began was the prevailing perception was that anthropogenic nitrate from the Woodside NW Shelf gas production facility might be accelerating the decay of rock art. Since the original surveys in 2003-2004 additional sources such as Yara Pilbara Nitrates (Yara), which produces ammonia and ammonium nitrate, have been added to the list of potential sources of pollutants. The 118 km2 of rugged landscape consists of boulder-strewn ridges and deep-sided valleys (Figure 7 & 8).

The Dampier Archipelago is on the Indian Ocean coast of the Pilbara region of Western Australia and the Burrup Peninsula was created by the bridging of Dampier Island, the largest of the 42 islands and islets, with the mainland. The petroglyphs were made by removing the outer deep red-brown weathered crust by percussion and scoring to reveal the pale colours of the parent gabbro and granophyre rocks within the sites. The contrast diminishes with time leaving the oldest as visually faded images, but still retaining the original relief. Dating of associated midden shells provides an age for the fully patinated engravings of 10,000–20,000 years. Engraved thylacines (Tasmanian tigers) date to more than 3,000 years ago (Figure 8) when the animals became extinct on the mainland of Australia. More recently sequential dating techniques revealed the earliest engravings to have been created around 40,000 years ago [17]. Gabbro rocks are rough-grained and have a characteristic crusty look while the granophyre rocks have a more uniform and fine-grained surface.
During the development of the Northwest Shelf Venture gas production facility in the 1980s, Woodside Petroleum relocated hundreds of engraved rocks to a ‘compound’ on the leeward side of the adjacent hills. The first in situ measurements on the ‘compound’ rocks and surrounding areas in 2003–2004 indicated significantly increased microbial activity associated with above background nitrate ion concentrations which were associated with decreased pH values and increased concentrations of metallic cations reporting to the washing solutions. The data also revealed that there was measurable loss of iron, manganese, and clay minerals from the most acidic surfaces [18]. The wash solutions were also analysed for sulphate, sulphite, nitrate, nitrite, and oxalate ions and the chloride concentrations and redox potentials on rock surfaces were measured.
Measurement of the rock surface pH, Eh and chloride concentration
One of the main differences between the gabbro and granophyre rocks at Murujuga is the concentration of calcium in their crusts with the former containing 10.9±1.9% CaO and the latter 1.4±0.8 %, which should be noted when comparisons are made between the wash solutions recovered from the two main rock types [18]. Data on fresh rainwater collected in the 2 km radius monitoring zone by Yara showed that pH is controlled by the buffering action of sea salts washed by rain into the collection receptacles. This relationship is shown in equation 5,
pH rain = 6.11 + 0.0079 [Na+] ppm (n=6, R2 = 0.92) (5)
The monitoring stations are also withing the air dispersion zones from the nearby Woodside plants which means it is not possible to readily identify the sources of the NOx. The amount of chloride provides evidence of the impact of the marine environment which indicates that salt weathering of rocks due to dehydration and rehydration cycling plays a significant role in the overall decay processes [23].The deposition of sea salt on the rock surfaces means that the CO32-/HCO3- buffer found in sea water reduces the variation in the pH of the microenvironment arising from a combination of microbiological and chemical reactions on the surfaces.
The pH at monitoring stations responds to nitrate, nitrite and to the ammonia emissions from the ammonium nitrate production facility and the data reveals both an abiotic and microflora mediated response. Separating these two influences, the abiotic contribution is a pH decrease of approximately 0.3/ppm nitrate, whilst biological activity after fresh rainfall results in a much more significant decrease of 1.2 pH units (at nitrate concentrations of only 0.16 ppm). This indicates that rock surface pHs at the sites are strongly influenced by the nature of the local microflora, itself determined by the availability of water and nitrate and nitrite concentrations. Because the local microenvironment is arid, the availability of moisture is a key limiting factor for both mechanisms and, despite low annual rainfall, damp or wet surfaces frequently develop for a few hours when a combination of low nighttime temperature and clear skies (allowing unimpeded heat loss) result in rock surface temperatures below dew point. Dissolved oxygen and an electrolyte composed of water and dissolved ions are essential components of electrochemically controlled oxidation and reduction reactions, and measurement of the electrical conductivity of the rock wash solutions shows that wind-borne salt spray aerosols provide sufficient electrolyte to promote corrosion reactions.
Owing to conceptual challenges in dealing with concentrations of cations as low as 10-7 M, and to more easily tease[1]out the relationship between a wide variety of mineral concentrations and the surface and solution pH, it is useful to report metal cations as pM values. Like pH values, pM values are -log10 [Mn+] in which higher pM values indicate less metal ion dissolution and a change of +1 unit being a ten-fold decrease. The weathering of the rock crusts involves many neutralisation (dissolution) reactions of either oxides or hydroxides of metal ions. The generic dissolution reaction can be written as in Equation 6,
M(OH)n + n H+ —> Mn+ + n H2O (6)
The n value is the oxidation state of the metal, typically 2 and 3 for iron and mixtures of 2, 3, 4 etc. for manganese. The metal ion concentration is derived from the general equilibrium constant for the dissolution of a metal hydroxide in water viz., Ksp = [OH-]n x [Mn+]. Rearranging this equation ,
1/Ksp = {1/[OH-]n} x {1/[Mn+].} (7)
and because the logarithm of {1/x} is pX, then the solubility equilibrium equation 7 can be expressed by the formula,
pKsp = n* p[OH] + p [Mn+] (8)
Equation 8 is further simplified using p[OH] = pKw – pH, where pKw =14, the self-ionisation constant of water. This leads to equation 9,
pMhydroxides = pKsp + n(pH-14) (9)
Plots of the pM values for metal ions have an intercept value (at zero pH) equal to (pKsp – n*14). For metal oxides of the general formula MxOy the concentration of dissolved metal in water is given by Equation 10 below,
pMoxides = 1/x {pKsp} + 2{y/x} pH (10)
When the pM values are plotted as a function of pH it is possible to determine the stoichiometry of the dissolution process since for a mixed valency oxide, the slope is 2y/x for the pM vs pH graph.
Mobilisation of metal cations
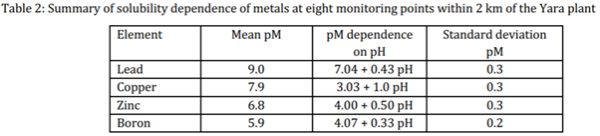
Eight months prior to field sampling in October 2020 cyclonic rainfall drove a general reduction in the mobilisation of transition and p-block metal ions, which originated from the weathered rock crusts. For lead (Pb) the mean concentration was approximately 10-9M (pM = 9.0) and the slope of 0.5pH for plots of pM vs. pH is consistent with dissolution of barstowite, Pb4Cl6(CO3).H2O, in which four lead ions are released for every two protons consumed (Table 2). Dissolution of copper minerals was low, as indicated by the mean pM Cu of 7.9 ± 0.3 which was significantly higher than lead. The 1:1 pM to pH slope is consistent with the dissolution of tenorite (CuO) from the rock surfaces. There was more zinc than copper released into the solution, with p[Zn] = 6.8, reflecting the expected differences in solubility of the lead, copper, and zinc minerals. The pM/pH slope of +0.5 for zinc implies its origin in mixed metal oxide and hydroxide species. Metal (and rock) corrosion is associated with concentrations of metal ions greater or equal to 1 x 10-6M (pM = 6) according to the Pourbaix Atlas of Electrochemical Equilibria [18]. The p-block metal boron had a mean concentration of pM of 5.90 in the sixteen rock washings collected on the eight sites in 2020 indicating a low rate of corrosion involving the metal probably due to the massive washing event during cyclone Damien eight months earlier in the year. When the log of the boron concentration was plotted against the pH, the slope was +⅓, consistent with the mobilisation of boron being dominated by the equilibrium between H3BO3 and HB4O7-, as shown in equation 11,
4 H3BO3 → HB4O7- + H+ + 5 H2O (11)
The mean ratio of chloride to boron in 2020 was 2,454 ± 434 and since this value is only 60% of the ratio in sea water, the extra boron results from the corrosion of granophyre and gabbro rocks. The main boron containing mineral in the Burrup rocks is chlorite, (Mg,Al,Fe,Li,Mn,Ni)4-6(Si,Al,B,Fe)4O10(OH.O)8 and this complex sheet silicate mineral is the most likely source of the elevated boron levels, which are acting as an indicator of its mobilisation or corrosion [19].
Dissolution of aluminium, iron, and manganese
The highly weathered gabbro and granophyre are characterized by a series of mixed amorphous iron—manganese oxides, in the form of desert or rock varnish, admixed with iron(III) oxy-hydroxides and weathered minerals such as smectite, kaolinite, illite and mica [19]. It was noted that during the more acidic 2003 and 2004 sampling periods, which were associated with higher nitrate and sulphate levels in the rock washing solutions, there was measurable mobilisation of aluminium (clays) iron and manganese containing minerals. Plots of the iron concentration as a function of the mean surface pH had an average slope of +1.98 ± 0.06 pH, implying the following mechanism:
FeOOH + 2H+ → Fe(OH) 2+ + H2O (12)
The Pourbaix diagram for iron in the pH range shows the Fe(OH)2+ ion to be the dominant form of soluble iron(III) under oxidizing conditions [20]. Under different environmental conditions the stoichiometry of the pM vs. pH plots reduced to 1:1 until the solubility plateaus at a mean pH of 5.6 recorded in November 2017. Kaolinite (Al2Si2O5(OH)4)was a major mineral identified in the CSIRO Accelerated Weathering experiments [23], and its mobilisation in acidic microenvironments was reflected in plots of aluminium concentration versus mean surface pH. The mean pM vs. pH slope was 1.4±0.2 pH, consistent with the dissolution of kaolinite according to Equation 13,
Al2Si2O5(OH)4+ 3H+ → Al(OH)2+ + AlSi2O5+ + 3 H2O (13)
In the aftermath of cyclone Damien, the October 2020 data showed that there was only one site (Yara East site 23b) with measurable aluminium in solution at 4.1 x 10-7 M, close to the detection limit of 1.9×10-7 M. The resetting of the surface environment in heavy rain to create a benign microenvironment for weathering of clay minerals supports the supposition that mobilisation of alumino-silicates (clays) is not a rock art management issue. The highest concentration of manganese was 2.0×10-7 M or a pMn of 6.70 at the Yara East (site 25) and the mean value for the pM vs. pH plots for sites 4, 5 and 22 was 1.09 ± 0.12, consistent with equation 14,
MnO + H+ → Mn(OH)+ (14)
The data was characterised by a very high correlation coefficient (R2 of 0.96) reflected in the small standard deviation of the slope. All the other sites had slopes of the p[Mn] vs. pH plots of 0.53 ± 0.10. In these cases, the dissolution of manganese from the rock surfaces follows the same pattern and mechanism but the stoichiometry is indicative, not of the dissolution of a distinct mineral phase on the rock crust, but of the dissolution of the rock varnish containing significant amounts of manganese.
Electrochemical characterisation of dissolution mechanism
Measurement of the redox potential of the rock surfaces began in 2018 following successful development of a suitable method whilst working on an early Bronze Age archaeological site in Kaman, Turkey [22]. The redox chemistry of manganese on the rock surfaces across Murujuga is complex which is in part simply due to the plethora of oxidation states in the natural environment.
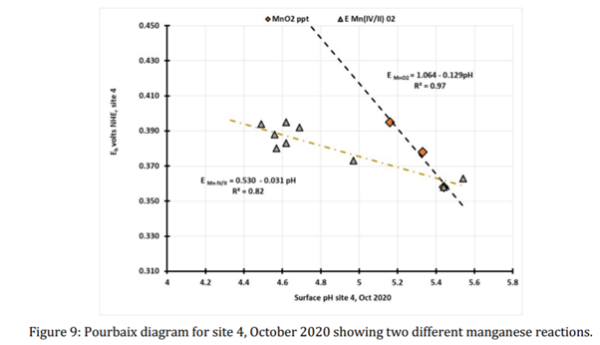
A typical set of results from site 4 at the head of the Climbing Man gully is illustrated by the Pourbaix diagram in Figure 9 in which the redox potentials are plotted against the surface pH. The slopes in Figure 9 reveal two distinct mechanisms controlling the precipitation of solid phases and dissolution at site 4. The steeper Eh/pH plot (equation 15) arises from the formation of insoluble MnO2, in which four protons are released and two electrons are consumed,
Mn2+ + 2 H2O → MnO2 + 4 H+ + 2e- (15)
For this reaction, the slope of -129 ± 10 mV/pH is consistent with the formation of insoluble manganese dioxide as the outcome of an oxidative hydrolysis and precipitation reaction. The standard redox voltage for equation 15 for a 10-6M Mn2+ solution is an E0 of +1.051, which is experimentally observed from the intercept value (at zero pH) obtained from regression analysis, which had the value of Eintercept of +1.064 volts at zero pH The Pourbaix plot with the lower Eh/pH slope is consistent with the oxidative hydrolysis of a partially hydrolysed Mn2+ ion, present as Mn(OH)+ to Mn(OH)22+ in Equation 16.
Mn(OH)+ + H2O → Mn(OH)22+ + H+ + 2e- (16)
The slope for equation 16 was -31. ± 5 mV/pH (R2=0.82) and the intercept voltage Eintercept was 0.530 ± 0.021 volts is consistent with a Mn(IV)/Mn(II) redox couple. Examination of the Pourbaix plots for all the monitoring sites in 2020 shows that all five granophyre sites exhibit this redox couple and that it is also found at the gabbro sites numbers 7 and 22.
The oxidation of Mn3O4 to Mn2O3 in equation 17 (Mn(III)/Mn(2.67) equilibrium) has a slope of – 59 mV/pH, which matched the experimental slope of -57 ± 4 mV (Eintercept = 0.718 ± 0.029 V) was obtained for the granophyre sites 4, 21, the Climbing Man split rock and for gabbro sites 7 and 23.
2 Mn3O4 + H2O → 3 Mn2O3 + 2 H+ + 2 e- (17)
The literature formal E0 of 0.689 volts for reaction 17 falls within one standard deviation of the mean of the intercept values for five of the rock art monitoring sites reflecting the expected range of conditions for exposed rocks in fully aerobic microenvironments.
The complexities of chloride distribution are illustrated by the salinity profiles (Figure 10) on the gabbro site 7, which sits high on a steep slope of tumbled cracked boulders with the right-hand side being closer to the sea. During the 2018 season it was noted that there was asymmetric distribution of chloride salts; the extreme left of the rock at all height levels was low in chloride (≤ 5 ppm). The highest concentration of chloride was found half-way down the rock surface (second row in Figure 11, upper plot in Figure 10) where night-time moisture condensation and wind had mobilised the salts away from the top edge concentrating them halfway down the 15-degree slope from the vertical. In this row there was a linear increase in chloride ion concentration (Equation 18) of 0.12 ppm/cm (R20.95) from below 5ppm up to a maximum of 11 ppm. [Cl] second row = 0.37 + 0.12 d (18)where d is the distance (cm) from the left side of the rock.
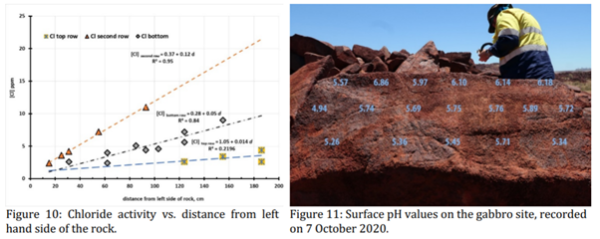
The [Cl-]vs distance slope of the second line of measurements is twice that of the bottom and 8½ times that of the top (Figure 10). Although the total amount of chloride deposited in the eight months since cyclone Damien is 1/20that found on site 6 which is 1.5 km closer to the coast, the pH gradient right to left across the rock in Figure 11 reflects the increasing alkalinity due to deposition of sea salts.
Effect of nitrates:
Analysis of variations in the mean annual pH readings across the many sampled sites in the Burrup showed a general trend for increased acidity with increased nitrate levels found in the rock washing solutions with the best data fit being nitrate concentrations in washings plotted against the minimum pH observed on the rock surfaces. Histograms of pH variation across the rock surfaces typically had a bimodal distribution centred on pH 4.4 and 5.6, the latter reading reflects the natural pH of the weathered rocks of the same geological origins and exposure environments. The aggregated nitrate data collected in 2021 was summarised in Equation 19,
pH minimum = 6.0 -1.6 [NO3]ppm (19)
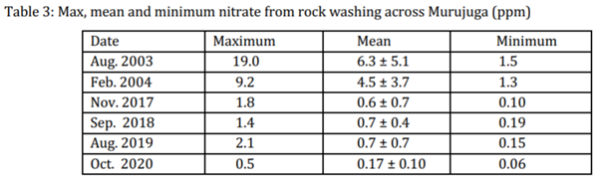
The sites captured in this relationship included the granophyre sites 5, 21, Climbing Man split rock and the gabbro sites at locations 7 and 23. Previous work by MacLeod had shown a direct relationship between the log of the bacterial counts and the amount of nitrate, with a concomitant decrease in the pH [18]. A summary of the relevant data is shown below in Table 3, which lists the mean nitrate for 2003, 2004, 2017 through to 2020 as well as the range of the maximum to the minimum values that were recorded.
CONCLUSIONS
The nature of the deterioration mechanisms in Western Australia affecting the alteration of pigments on rock art sites has been determined using applied micrometeorology, mineralogy, and electrochemistry over the past 40 years. Having established the deterioration mechanisms the traditional owners and government bodies involved in heritage management of Aboriginal sites now can review the relevant literature and make informed decisions on interventions that might slow down the rate of decay on specific sites. The cooperation of the traditional owners in the Murchison, the Pilbara and the Kimberley regions has been fundamentally significant to the success of the programs, which were developed to improve the management of rock art sites for the Western Australian Museum and for industries in the Pilbara. There is a large amount of work to be done with isotope measurements on the nitrates found on the rock surfaces to determine the relative significance of natural and anthropogenic sources. Improved emissions control from all local industries in the Pilbara will ensure that their presence will have a minimum impact on the long-term deterioration of engraved rock art sites.
ACKNOWLEDGMENTS
My colleagues Philip Haydock and Bruce Ford have been instrumental in guiding me down the right path of conservation of Aboriginal cultural materials. Field work has been conducted during extremes of weather in often very remote locations but experiencing the Kimberley sites in both the wet and dry seasons was a key to our success. X-ray diffraction data has been collected by colleagues at the ANU, CSIRO mineralogy at Floreat and Kensington and at the Chemistry Centre of Western Australia. The Western Australian Heritage Council and the Western Australian Heritage Commission and Yara Pilbara Nitrates all provided funding in addition to the original Pilbara studies which were funded by the Registrar of Aboriginal Sites. The Murujuga Aboriginal Corporation were most gracious with the cooperation and logistical support during the Pilbara field work. Bruce Ford greatly assisted with editing this paper. Friends of Australian Rock Art (FARA) provided funding for field work that reactivated the research project after a dozen years.
REFERENCES
- Mowaljarlai, D, 1985, “Bible in stone”, Personal communication.
- MacLeod, I.D., & W. Fish. 2021. Determining decay mechanisms on engraved rock art sites using pH, chloride ion and redox measurements with an assessment of the impact of cyclones, sea salt and nitrate ions on acidity. In Transcending Boundaries: Integrated Approaches to Conservation. ICOM-CC 19th Triennial Conference Preprints, Beijing, 17–21 May 2021, ed. J. Bridgland. Paris: International Council of Museums, 1-9.
- North, N & J. Clarke, 1987, Conservation of Post-Estuarine Period Rock Art in Kakadu National Park, Report on Phase One Study: Pigment Identification. Unpublished report, Australian National Parks and Wildlife Service.
- Haydock, P., & J Rodda., 1986, A survey of rock art conservation in the Murchison/Wheat belt area of W.A.: A study of past treatments and new methods of measurement and site management. Unpublished report to the Western Australian Heritage Committee. pp 1-45.
- Bureau of Meteorology, Climatological Records up to 1991. Bureau of Meteorology, Perth.
- Haydock, P. & I.D. MacLeod, 1987, The use of micro-meteorological studies as an aid to the conservation of aboriginal rock art, ICOM Committee for Conservation, 8th Triennial Meeting, Sydney, September 1987, Volume III, p 1149-1153.
- MacLeod, I.D., P. Haydock, & E. Charton, 1996, Avian guano and its effects on the preservation of rock paintings, Preservation of Rock Art 1995 AURA Occasional Papers 9, Ed. Andrew Thorn & Jacques Brunet p. 60-64.
- Deer, W.A., , R.A Howie. & J. Zussman, ,1971. Rock Forming Minerals Vol.3 Sheet Silicates. Longman, London.
- Tworek-Matuszkiewicz, B., 2007, Australian Aboriginal bark paintings – their history, structure and conservation, Studies in Conservation, 52 :sup1, 15-28.
- Clarke, J., 1976, Two Aboriginal rock art pigments from Western Australia: their properties, use and durability, Studies in Conservation, 21, 134-142.
- Ford, B., I.D. MacLeod & P. Haydock, 1994, Rock art pigments from the Kimberley region of Western Australia: identification of the minerals and conversion mechanisms”, Studies in Conservation 39, p 57-69.
- Watchman, A.L., 1990, A summary of occurrences of oxalate-rich crusts in Australia, Rock Art Research 7(1) 44-50.
- del Monte, M and Sabbioni, C., 1983, Weddellite on limestone in the Venice environment’ Environmental Science and Technology 17, 518-522.
- Wiedmann, H.G. & G., Bayer, 1989, Formation of whewellite and weddellite by displacement reactions, in The Oxalate Films: Origin and Significance in the Conservation of Works of Art, Milan, 127-131.
- MacLeod, I.D., P. Haydock, D. Tulloch, and B. Ford, 1995. Effects of microbiological activity on the conservation of aboriginal rock art, AICCM Bulletin 21(1): 3–10.
- Bird, C., and S.J. Hallam, 2006, Archaeology and rock art in the Dampier Archipelago. Unpublished report to the National Trust of Australia (WA), 1–27.
- Mulvaney, K., 2011, About time: Towards a sequencing of the Dampier Archipelago petroglyphs of the Pilbara region, Western Australia, In Records of the Western Australian Museum Supplement 79 (“Fire and hearth” forty years on: Essays in honour of Sylvia J. Hallam): 30–49.
- MacLeod, I.D.,2005, The effects of moisture, micronutrient supplies and microbiological activity on the surface pH of rocks in the Burrup peninsula, Preprints for ICOM-CC Triennial Meeting, Den Haag, The Netherlands, September 2005, Vol II 386-393.
- Ramanaidou, E., G. Walton, and D. Winchester, 2017, Extreme weathering experiments on the Burrup Peninsula/Murujuga weathered gabbros and granophyres, Report EP172193, 1–67. Canberra: CSIRO Mineral Resources.
- Pourbaix, M., 1974, Atlas of electrochemical equilibria in aqueous solutions, trans. James A. Franklin (except Sections I, III 5 and III 6, which were originally written in English), 2nd ed., 286–93. Houston/Brussels: National Association of Corrosion Engineers (NACE)/CEBELCOR.
- Lau, D., E. Ramanaidou, S. Furman, A. Hacket, M. Caccetta, M. Wells, & B. McDonald. 2008. Burrup Peninsula Aboriginal petroglyphs: Colour change and spectral mineralogy 2004–2007, 1–44. Clayton: CSIRO Materials Science and Engineering.
- MacLeod, I.D., & A.B. Paterakis, 2021, Bronze corrosion on archaeological sites: Correlation of past and present microenvironments via in situ pH, chloride, and redox measurements. In Transcending Boundaries: Integrated Approaches to Conservation. ICOM-CC 19th Triennial Conference Preprints, Beijing, 17–21 May 2021, ed. J. Bridgland. Paris: International Council of Museums, 1-8.
- MacLeod, I.D., 2004, The microenvironment of rocks on the Burrup: analysis of the relationships between nutrient supplies, surface pH and microflora”, Un published report to the Minister of Culture and the Arts, pp 1-63.
AUTHOR DETAILS
Dr Ian D. MacLeod AM worked for the Western Australian Museum from 1978-2016 studying corrosion of metals on historic shipwrecks. A former Executive Director of the WA Maritime Museum he now runs Heritage Conservation Solutions. He was editor of Corrosion and Materials for seven years and is currently a director of the ACA Foundation. He was awarded The Corrosion Medal in 2004 and became a Life Member in 2014. He has been the P.F. Thompson lecturer on three occasions and has published more than 290 papers on materials conservation and corrosion. He was awarded his DSc in 2007 for his work on conservation of shipwrecks and rock art.