Authors: C. Özkan, J.M.C. Mol
This paper was presented at Corrosion & Prevention 2023.
ABSTRACT
The research on active coatings for corrosion protection has recently witnessed a surge, driven by the non-negotiable solutions required to meet enhanced industrial needs as well as strict health and safety regulations. Our lab has been involved in many recent developments of active coatings, where we contributed to the mechanistic understanding of corrosion and its inhibition, with a particular attention to the replacement of hexavalent chromium chemistries in the protection of aerospace alloys. Leveraging a wide variety of advanced analysis methods, including various electron microscopic, electrochemical and spectroscopic techniques, we uncovered macro- and microscale corrosion mechanisms of metal alloys and the active protective and self-healing performance of coatings. Besides of studying many other inhibitor chemistries, a particular research interest of ours is exploring lithium salts as chromate substitutes, which revealed their potential of developing into a commercial solution the industry has been looking for. On another side we’ve been trying to find better screening methods for organic molecules suitable for corrosion inhibition, through high-throughput screening and machine learning methods, which hold promise in the discovery and optimization of corrosion inhibitors by providing a pathway towards a more systematic approach to materials discovery. Through a comprehensive experimental library of small organic molecules, and development of preliminary predictive models, we lay the foundation for future research in the realm of corrosion inhibition and active coating development. Our hope is that our contributions will steer us towards a future where the scientific community can rapidly and efficiently navigate the vast chemical space in search of optimal corrosion inhibitors.
Keywords: active coatings, corrosion inhibitor, aerospace aluminium alloys, Ce, Li, TEM, machine learning
Materials Science of Active Coatings
Recent years have witnessed a surge in research activity related to coatings, driven heavily by industrial and societal grand challenges. Critical questions focused on enhancing energy efficiency and reducing CO2 emissions, as well as finding alternatives to the use of toxic hexavalent chromium chemistries. The search for novel alternate inhibitors, encapsulation methods, delivery systems, coating matrix structures, pre-treatment processes resulted in active coating systems that can respond to the environment. Coatings that can sense and signal environmental changes, or even self-heal by closing cracks in the polymer matrix, or hinder corrosion reactions by leached corrosion inhibitors became a practical reality.
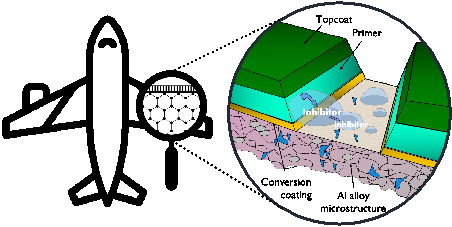
Figure 1. Active protective coatings system for an aerospace alloy that consists of a topcoat, primer, anodized layer and the underlying substrate. In the presence of a coating defect corrosion inhibitors leach from the primer to hinder corrosion reactions. Adapted from Scholes et al. 1.
For decades, chromate-based coatings have been vital for protecting high-strength aluminium alloys due to their exceptional performance and self-healing properties in corrosion prevention. Chromates are present in many stages of corrosion protection: pre-cleaning, anodizing, and organic primer coatings. In the presence of a layer defect active protection can be provided through corrosion inhibitor-substrate interaction. While hexavalent chromium offers unique, hard-to-replace inhibitive properties, extensive research has been conducted to find alternate compounds that provide similar corrosion protection and self-healing capabilities, critical for safeguarding aluminium alloys. Ideal corrosion inhibitor compounds should be released at just the right amount from the reservoir, also at suitable release rates: not too fast so it would be depleted and dissolved into the exposed electrolyte, not too slow so corrosion would progress to catastrophic levels, but the right amount that ensures good interaction with the exposed metal/oxide surface to create a protective layer. It also should naturally inhibit corrosion reactions, and should not be interacting with the matrix that it is carried in, for example it shouldn’t interact harmfully with the organic coating chemistry.
The critical need for replacing hexavalent chromium chemistries from active corrosion protection of aerospace alloys has driven the corrosion protection research for the legacy alloy AA2024-T3, which has been the focus of our research and development over the last few decades. We’re still far away from a definite, one-fits-all commercially usable next generation active protective coating system, but research has come far in finding alternatives. Through new techniques, materials and approaches, our lab has been an active contributor to the fundamental findings in this field.
Seeing Corrosion and Inhibition through TEM
Understanding starts by seeing. Through electrochemistry, electron microscopy and various spectroscopies, we’ve uncovered the mechanisms of corrosion and its inhibition for many material-environment systems. However, for a long time we’ve lacked ‘seeing’ the actual corrosion at the required spatial and time resolution, due to its atomic nature and rapidity of reactions.
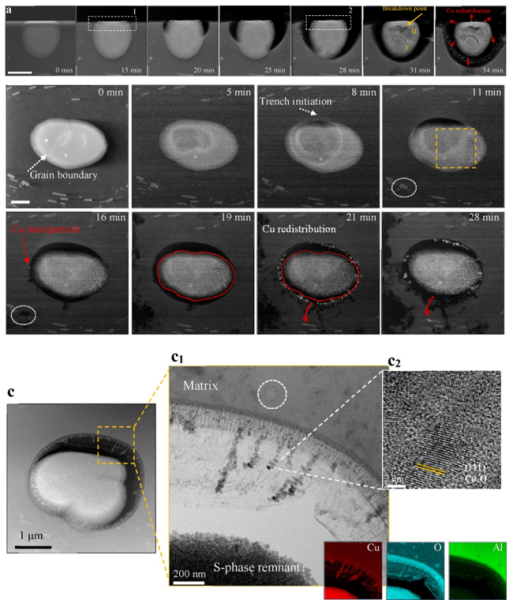
Figure 2. In-situ cross-sectional and top-view observations of an AA2024-T3 S-phase particle, and STEM/EDS analysis of the corroded sample revealing selective dissolution of Al and Mg to different extents2.
To tackle that, we’ve created nanometric sandwiched thin specimens with liquid cells designed for application in liquid-phase transmission electron microscopy (TEM) corrosion studies. With an in/ex-situ analytical TEM investigation of Al2CuMg and Al2Cu precipitates we have observed a dealloying driven local corrosion mechanism2. Local corrosion starts with a surface initiation stage, where a passive layer covering the alloy destabilizes and locally dissolves at and around the intermetallic. Mg/Al dissolve and hydrolyse from the intermetallics, meanwhile Cu diffuses to the surface rim of the pits. Al2Cu exhibits a relatively slower corrosion initiation as a consequence of formed Al(OH)3. Nanogalvanic interactions form inside the intermetallic, and Cu rich cathodic sites start producing OH–. This makes the local surface atop intermetallics alkaline. Due to faster dealloying, local chemistry becomes more alkaline for Al2CuMg. The increase in pH locally dissolves the surrounding Al oxide passive layer. This triggers trench initiation. Pits start to propagate in depth of the matrix: cathodic oxygen reduction reaction (ORR) take place on the surface of the intermetallics while the Al matrix around the intermetallic dissolve until the particle is undercut. When the remaining Cu rich intermetallic remnant is undercut, Cu will undergo self-corrosion, dissolves into the solution and gets redeposited on the Al matrix or other intermetallic surfaces. Cu replating can create more cathodic areas and promote further localized corrosion of the surrounding Al matrix. Due to faster kinetics of the Al2CuMg, Cu ions liberated earlier from its dissolution can be redeposited onto the Al2Cu precipitates and other intermetallics.
With a follow-up TEM study3 we’ve demonstrated similar degradation mechanisms for isolated constituent particles Al76Cu6Fe7Mn5Si6, Al7Cu2Fe(Mn). Nano-pits initiate with dealloying attack of active elements Al, Mg, Mn while Cu and Fe rich cathodic zones undertake the reduction of oxygen. Local dissolution rate increases with increasing exposure. Dealloyed zones of the intermetallic become more cathodic, and start the trenching through dissolving surrounding Al matrix. Depth propagation occurs after trench initiation at the area surrounding the intermetallic particles. It is seen that Si inhibits the reaction through oxidizing into stable SiO2, while Mn actively dissolves away. Due to the higher electrochemical stability of constituent particles than the Al2CuMg precipitate, copper ions released during the Al2CuMg corrosion may deposit onto the constituent particles in vicinity. This would increase the cathodic activity of intermetallic particles and increase local dissolution.
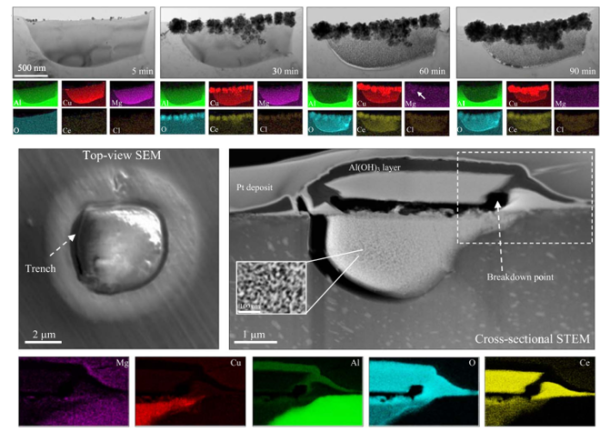
Figure 3. Time-resolved TEM images and the corresponding EDS maps of an S-phase particle, and STEM/EDS analysis of the nanoporous morphology of precipitated Ce cover4.
Dispersoid particles Al20Mn3Cu2 exhibit a similar dealloying driven local corrosion behaviour to the constituent particles, although on a slower time and a smaller length scale3. In fact, a recent analysis of AA2024 microstructure with OCP and optical microscopy suggest that all intermetallic phases show similar micro/nanogalvanic activation, dealloying and trenching behaviour5. Main difference between the intermetallic particles was found in the dealloying step – trenching occurred at similar rates independently from intermetallic composition. On the other hand, it is reported that compared to Al-Cu-Mn-Fe phases, Al-Cu-Mg phases account for most of the increase in ORR on AA2024 relative to pure Al6.
Investigation of Ce(NO3)3 exposed samples at nanoscale showed that in contrast to micro-galvanic corrosion between intermetallic particles and the surrounding matrix, dealloying of intermetallic particles acted as the main factor governing the rate of local cerium precipitation – the faster the dealloying the more cerium precipitation. Local alkalinity resulting from anodic dealloying reactions was the key, which triggers cerium precipitation. Furthermore we confirmed that intermetallic particles, regardless of their conventional categorisation, are in fact simultaneous anodes and cathodes as from the beginning of the exposure4.
Studies of lithium deserves its own background as we’ve been involved in many aspects of the mechanistic understanding of lithium inhibition technology.
Lithium, A New Hope?
Lithium (Li) salts as potential chromate substitutes have initially been proposed in the 90s, where alkaline Li electrolytes were explored as replacements to the conventional chromate chemical conversion processes. Li salts have been shown to produce a continuous polycrystalline layer on pure Al and its various alloys, which resulted in increased pitting potentials and decreased corrosion current densities7,8.
To gain a mechanistic understanding of the protection offered by Li, we have characterized the formation process through several techniques. We have looked at both bare surfaces exposed to electrolytes, and leachable Li salts incorporated into coatings. Li carbonate and oxalate loaded polyurethane coatings exposed to corrosive conditions like 168 h neutral salt spray (ASTM B-117) resulted in clean scribes with no corrosion products. Leached Li ions formed a 0.5 – 1.5 um thick layer with three distinct layers visible with SEM: a dense barrier layer near the metal/passive layer interface, a porous layer in the middle, and an outer columnar layer9,10. Electrochemical techniques confirmed irreversible long-term corrosion resistant properties of these layers11,12. Coating leaching profiles revealed an immediate release of Li ions from the coating matrix into the defect under corrosive conditions, elevating the pH to alkaline levels between 9 and 10. XPS and Auger analyses indicated no intercalation of carbonate or oxalate ions in the protective layer, suggesting their role is primarily for pH buffering. The layer’s surface primarily consisted of Al and O with traces of Li and Mg, where the layer was pseudoboehmite (hydrated Al oxide) with 1–2 at.% Li, rather than a Li-based layered double hydroxide13.
The formation of such a protective layer is based on a multistep film growth and dissolution process. The formation consists of four stages: (I) Li-ion release upon environmental exposure, (II) oxide thinning and anodic dissolution initiating protective layer formation, (III) defect coverage, and (IV) layer growth14. In the initial stages the dominant localized electrochemical reactions occurring simultaneously are hydrogen evolution, trenching of the matrix and dealloying of intermetallic particles. Afterwards, the growth of a passive conversion layer becomes the dominant process15. During the conversion layer evolution process, the alloy surface experiences a transition from insufficient passivation to activation and then back to passivation16.
To develop a complete mechanistic understanding of this conversion layer formation characteristics we’ve performed TEM analytical studies. At early stages of exposure, passive layer and alloy matrix dissolves due to increased alkalinity, leading to surface Cu enrichment and S-phase dealloying. These precede the formation of a columnar layer on the alloy, followed by formation of a dense-like layer. Enhanced barrier properties were the result of this dense-like layer with low porosity. Outer columnar layer is composed of mostly crystalline Li–Al layered double hydroxide and was the result of higher pH and Li concentration. This was the intercalation product of CO32−, H2O and even Cl− ions with the d(003) planes containing Al3+, Li+ and OH−, which leads to a sandwich-like structure. On the other hand, the 100-150 nm protective dense-like inner layer contained amorphous Li pseudoboehmite. In between porous layer was a mixture of both crystalline and amorphous structure. With time, the needle-like formation morphology grows taller and denser. Dealloying of intermetallics such as S-phase can assist the conversion process by providing supersaturation of Al(OH)4–, which was critical for formation of the conversion layer. Through complementary experiments in a sodium carbonate solution and additional X-ray diffraction analysis, Li was shown to play a critical role in stabilizing the corrosion product throughout the conversion process17–21.
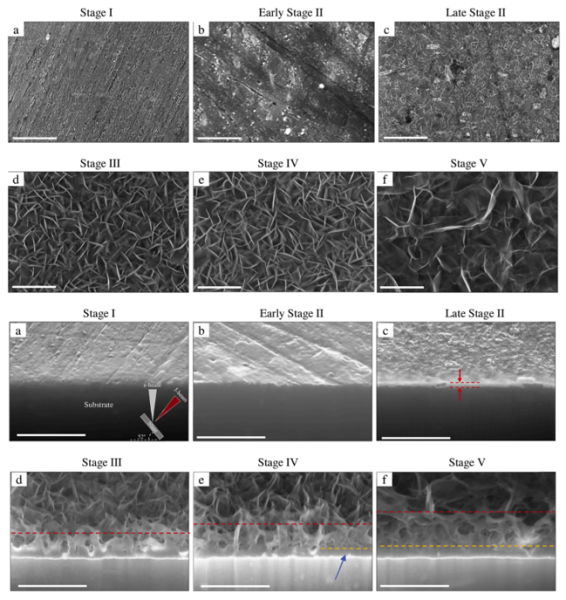
Figure 4. Time-resolved TEM images of the Li conversion layer growth (a) 60 s; (b) 200 s; (c) 800 s; (d) 2800s; (e) 2 h; (f) 7 h. The scale bar represents 1 μm. The red dashed lines indicate the highest location in the conversion layer at each stage. The orange dashed line separates the columnar layer from the dense-like layer17.
The stability of the protection depends on the mode of layer formation. Conversion layers aged for short durations initially exhibit high corrosion inhibition, which significantly diminishes after the inhibition stage ends. In contrast, layers aged for longer periods demonstrate relatively stable and superior corrosion resistance compared to their less aged counterparts. Initially, the freshly-formed Li-based layer entraps water and Li ions, which integrates gradually into the conversion layer during ageing. The fresh layer temporarily grants a high corrosion resistance and exhibits active inhibition by leaching entrapped Li carbonate in early re-immersion. While long-term ageing reduces the initial active protection capability, it enhances the overall corrosion barrier resistance of the conversion layer due to its continuous development during ageing22. Protection is also dependent on Li concentration during transformation. It was shown that active Li containing primers were able to protect coating defects with a width up to 6 mm23.
To understand the behavior of Li leaching from a Li containing primer, we have analyzed the ion transport pathway development from the scribe edge of a primer/topcoat system. X-ray emission spectroscopies of Li containing primers show that Li is locally concentrated into small regions around 7 μm in size, reflecting the presence of particles. These particles can be further interconnected, suggesting clusters of Li carbonate24. After exposure to neutral salt spray, a leached zone originating from the scribe edge with almost complete Li and Mg depletion was observed. However homogenous leaching did not extend beyond the scribed area even with prolonged exposure, suggesting it resulted from mechanical scribing damage. From the electrolyte/primer interface a homogenous depleted zone of around 10 μm extended into the primer. A deeper local penetration was observed, which was the result of voids created by partial dissolution of Li carbonate particles. Initially, small voids formed in particles distant from the scribe (100–250 μm), whereas a combination of voids and detachment around particles was observed with longer exposure. The hypothesis was that the detachment was part of a particle cluster channel network. To test this, FEM analysis of internal primer stresses arising from inhibitor dissolution product accumulation in the voids was performed. Internal stresses were focused around inorganic particles and within the primer plane beneath the topcoat, mainly concentrating towards the primer/metal interface, which corresponded to the cracks of the binder observed during experiments. Leaching experiments revealed that the Li release from the primer follows a power law where kinetic behavior depends on primer porosity, which resulted in a rapid initial Li release very quickly becoming sluggish25,26.
High Throughput Screening of Corrosion Inhibitors
Serendipity, coupled with curiosity and observation, has played a significant role in the discovery of new materials – see the discovery of Teflon, cellophane, vulcanized rubber, polyethylene, safety glass, super-glue, among many others. Discovery process of lithium as a corrosion inhibitor was also jokingly described by Peter Visser as an “opportunistic literature search during lunch”27. This begs the question: can we accelerate the material discovery process, and make discovery more structural and less chance based?
Enter high-throughput screening (HTS). HTS methods have played a pivotal role in the field of drug discovery, particularly in the identification of active small molecules. The advent of these methods can be traced back to the late 20th century, with significant advancements occurring in the 1980s and 1990s. The development of HTS was driven by the need to accelerate the drug discovery process, and to efficiently screen vast libraries of compounds against biological targets. The combination of advancements in robotics, automation, and informatics enabled the management and interpretation of large datasets generated by HTS. Result was a billion dollar industry that supports the increasing demands for speed, capacity and cost effective screening of vast libraries of compounds28.
HTS has rapidly been taken in by diverse research fields, although corrosion science was a late adopter. However, HTS of corrosion inhibitor candidates is critical for rapid progress because corrosion testing, not chemical synthesis, is the rate-determining step in corrosion inhibitor discovery. In the past 15 years encouraging progress has been made in high-throughput characterisation of corrosion inhibition, which we’ve also contributed with new HTS techniques. Our previous approaches are visible in Figure 5. In one setup we explored testing pairs of different metal electrodes immersed in the same inhibitor solution, where a constant imposed potential applied between identical metal pairs allowed 30 electrochemical experiments to be performed per hour, without the use of a reference electrode. This configuration allowed rapid identification of inhibitor influence on various metal electrodes, and the rapid detection of the optimal pH and inhibitor concentration29,30. Another setup that we’ve also utilized assessed the potential of a well configuration, where a sheet metal was covered with multiple inhibitor electrolyte containing wells. In this way 88 experiments can be performed in a single day, without the need of any potentiostats. Optical analysis was automated to correlate images of corroded samples to results of standard 28 day immersion mass loss experiments, which enabled semi-quantification of the optical corrosion data31. We also employed a spectroscopic approach to study metal dissolution. ICP-AES Al and Cu signals, visual inspection and image analysis of pits were used to investigate performance of AA2024 corrosion inhibitors during flowing electrolyte conditions for 10 parallel channels. Microfluidic multi-channel assays, where solutions flown over the surface of a metal accelerated corrosion up to 15 times when compared to standard immersion tests. Faster testing enables rapid screening, both in the sense of shorter experimentation times and investigation of different chemistries through parallel experimentation32.
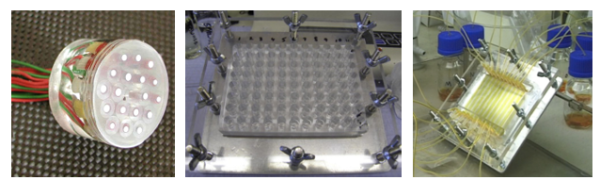
Figure 5. Experimental high-throughput corrosion inhibitor screening setups. Left: Multi-electrode assembly showing the layout of wire specimens 28,29. Middle: Multi-well corrosion inhibition setup 30. Right: Multi-channel microfluidic arrays 31.
Laying The Experimental Foundation For Machine Learning Predictions
Our HTS approaches have shown potential in enabling predictive machine learning (ML) models for corrosion inhibitors, where discovery of small organic molecules can be progressed rapidly in-silico33. Meanwhile our other works on the influence of molecule structure on inhibition34, and the dependency of inhibition on time35, have shown the importance of a robust electrochemical foundation.
A recent review has highlighted that the main challenge of utilising ML for corrosion research is the lack of high-quality datasets36 .Electrochemical data is generated with different electrochemical techniques at different times of electrolyte exposure with different sample preparation procedures. This complicates combining and comparing the results from separate studies into one cohesive picture. To resolve this problem, we have created an extensive electrochemical library of more than 100 inhibitor candidate small organic molecules. The electrochemical behaviour of inhibitor exposed AA2024-T3 substrates was captured using linear polarisation resistance, electrochemical impedance spectroscopy, and potentiodynamic polarisation techniques at different timesteps to obtain the most comprehensive electrochemical picture of the inhibition in the first 24 hours.
For initial inhibitor screening purposes, time-weighted LPR measurements showed very high correlations with other techniques and are a good metric for representing the protective behaviour of the inhibitor. Although we need to be careful with quick observations: we’ve observed that measurements of most of the organic molecules performed in less than 6 hours varied in time and were unstable. To have reliable measurements and understand the true inhibitive properties of inhibitor candidates, electrochemical studies should be performed at least after 6 hours.
Representation of corrosion inhibition is also important. Statistical analysis shows that inhibition efficiency may not be an “efficient” way to distinguish between good inhibitors. We believe that inhibition power is a more suitable metric for discerning between “better” and “best” inhibitors. Inhibition power eliminates clustering of data observed in higher efficiency range (>90%), which is important in identifying differences between the best inhibitors, and also an important condition for training an unbiased machine learning model.
On the machine learning side, at this stage rather than designing a final prediction system, we have explored the use of machine learning models to create an active learning loop for more efficient experimental discovery. Our initial explorations show that models augmented with mechanistic information is key in exploring the complexity of corrosion phenomena, which was highlighted by the predictive power of pH. Despite no observed linear relationship between bulk pH and inhibitor performance, information gained from pH assisted in describing the system better by including information about the environment not necessarily found in computational descriptors, which increased the prediction rate and assisted in outlier analysis of the random forest models. Such input features exhibit great potential to develop augmented quantitative structure-activity relationships as they allow the direct inclusion of information about the underlying mechanisms in training of the models. This can support the development of faster inhibitor screening techniques in the future, which can leverage the link between the molecular structure of the inhibitor and its protective performance.
Representation Matters: Describing Inhibition
Major breakthroughs in science often went hand-in-hand with new mathematical notations. A good example of this is Newton and Leibniz’s definition of derivatives – which started with a need from the scientific problem itself and afterwards this mathematical foundation enabled easier understanding of and solving a myriad of problems. Language of quantum mechanics also started with a new mathematical notation, which changed the understanding of all physical sciences. In a similar manner, currently we are lacking the optimum notation, the description needed to digitize our experiments. This is critical in communicating our results to come up with predictive machine learning models. For this end, right now we are working on finding the computational descriptors that best digitize corrosion inhibition.
Literature contains many descriptor generation packages for quantitative-activity relationships (RDkit, Dscribe37, etc.), and promising approaches that move beyond externally generated features by intrinsically defining molecules (graph neural networks, adjacency matrices, natural language embeddings of SMILES/SMARTS/SELFIES). By understanding the relationship between different descriptions and our experimental results, we hope to come up with the language of corrosion inhibition phenomena. At this stage this is undertaken solely for small organic molecules, but a future expansion of this would enable us to capture the behaviour of inorganic inhibition systems such as protection offered by Li, Ce, chromate systems, and further down the line interaction between different inhibitor chemicals, and interaction of different components in the coating system. However, coming up with such a unified language is highly non-trivial.
Where to Go From Here
We believe that the next step in active coatings should blend a solid description of the corrosion inhibition, an experimental foundation that offers mechanistic insights, high-throughput experiments that facilitate the screening process, statistical models that facilitate experimental and computational investigations through active learning, and robust in-silico screening through machine learning models to explore the vastness of the chemical space. Examples from other fields have shown inspiring approaches to similar problems we today face; such an approach is shown in Figure 6.
Some interesting open questions to explore:
- Defining features that describe the active protection system: for substrate chemistry and microstructure; pre-treatment of the substrate; interactions of corrosion inhibitors with the surface, one another in mixtures and with additives together with the polymer matrix.
- Creating high-throughput setups for coating characterisation: inhibitor systems with ternary and more combinations, coatings with gradients.
- Creating the perfect materials loop: experiments to DFT models to machine learning predictions – self driving labs for inhibitor discovery.
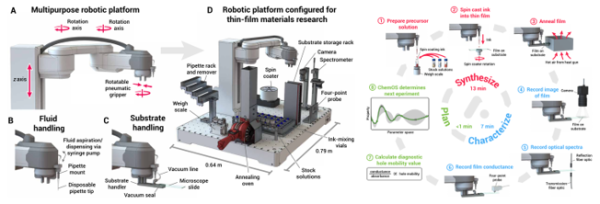
Figure 6. A self-driving laboratory for synthesis and characterization of thin-film materials38.
Acknowledgments
This work is a compilation of parts of a range of previous research projects and outcomes of the invaluable hard work of many colleague researchers. The authors acknowledge the IOP-Innovatiegerichte Onderzoeks-Programma’s (Innovative Research Program) in The Netherlands for this research under Project No. IOP-SHM0633; the research programme Understanding Processes using Operando Nanoscopy (UPON) with project number 14205 (B2), which was financed by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) and partly by AkzoNobel; the collaboration agreement between Delft University of Technology and AkzoNobel on the Li-based inhibitor and protective coating technology; the VIPCOAT project (Virtual Open Innovation Platform for Active Protective Coatings Guided by Modelling and Optimisation) funded by Horizon 2020 research and innovation programme of the European Union by grant agreement no. 952903.
Arjan Mol wishes to dedicate this contribution to two of his lifetime mentors, Dr Bruce Hinton (DSTO) and Dr Tony Hughes (CSIRO), who have guided him professionally and personally during and as from the early stages of his academic career and to whom he is immensely grateful and indebted.
References
- Scholes, F. H., Furman, S. A., Hughes, A. E. & Markley, T. A. Corrosion in artificial defects. I: Development of corrosion. Corros. Sci. 48, 1812–1826 (2006).
- Kosari, A. et al. In-situ nanoscopic observations of dealloying-driven local corrosion from surface initiation to in-depth propagation. Corros. Sci. 177, 108912 (2020).
- Kosari, A. et al. Dealloying-driven local corrosion by intermetallic constituent particles and dispersoids in aerospace aluminium alloys. Corros. Sci. 177, 108947 (2020).
- Kosari, A. et al. Editors’ Choice—Dealloying-Driven Cerium Precipitation on Intermetallic Particles in Aerospace Aluminium Alloys. J. Electrochem. Soc. 168, 041505 (2021).
- Olgiati, M., Denissen, P. J. & Garcia, S. J. When all intermetallics dealloy in AA2024-T3: quantifying early stage intermetallic corrosion kinetics under immersion. Corros. Sci. 109836 (2021) doi:10.1016/j.corsci.2021.109836.
- Jakab, M. A., Presuel-Moreno, F. & Scully, J. R. Effect of Molybdate, Cerium, and Cobalt Ions on the Oxygen Reduction Reaction on AA2024-T3 and Selected Intermetallics. J. Electrochem. Soc. 153, B244 (2006).
- Rangel, C. M. & Travassos, M. A. The passivation of aluminium in lithium carbonate/bicarbonate solutions. Corros. Sci. 33, 327–343 (1992).
- Drewien, C. A., Eatough, M. O., Tallant, D. R., Hills, C. R. & Buchheit, R. G. Lithium-aluminum-carbonate-hydroxide hydrate coatings on aluminum alloys: Composition, structure, and processing bath chemistry. J. Mater. Res. 11, 1507–1513 (1996).
- Visser, P. et al. The corrosion protection of AA2024-T3 aluminium alloy by leaching of lithium-containing salts from organic coatings. Faraday Discuss. 180, 511–526 (2015).
- Liu, Y. et al. Protective Film Formation on AA2024-T3 Aluminum Alloy by Leaching of Lithium Carbonate from an Organic Coating. J. Electrochem. Soc. 163, C45–C53 (2016).
- Visser, P., Meeusen, M., Gonzalez-Garcia, Y., Terryn, H. & Mol, J. M. C. Electrochemical Evaluation of Corrosion Inhibiting Layers Formed in a Defect from Lithium-Leaching Organic Coatings. J. Electrochem. Soc. 164, C396–C406 (2017).
- Visser, P., Terryn, H. & Mol, J. M. C. On the importance of irreversibility of corrosion inhibitors for active coating protection of AA2024-T3. Corros. Sci. 140, 272–285 (2018).
- Visser, P., Lutz, A., Mol, J. M. C. & Terryn, H. Study of the formation of a protective layer in a defect from lithium-leaching organic coatings. Prog. Org. Coatings 99, 80–90 (2016).
- Visser, P., Gonzalez-Garcia, Y., Mol, J. M. C. & Terryn, H. Mechanism of Passive Layer Formation on AA2024-T3 from Alkaline Lithium Carbonate Solutions in the Presence of Sodium Chloride. J. Electrochem. Soc. 165, C60–C70 (2018).
- Li, Z. et al. Evaluation of the formation and protectiveness of a lithium-based conversion layer using electrochemical noise. Electrochim. Acta 426, 140733 (2022).
- Li, Z. et al. Local scanning electrochemical microscopy analysis of a lithium-based conversion layer on AA2024-T3 at progressive stages of formation. Electrochim. Acta 469, 143270 (2023).
- Kosari, A. et al. Laterally-resolved formation mechanism of a lithium-based conversion layer at the matrix and intermetallic particles in aerospace aluminium alloys. Corros. Sci. 190, 109651 (2021).
- Kosari, A. et al. Nanoscopic and in-situ cross-sectional observations of Li-based conversion coating formation using liquid-phase TEM. npj Mater. Degrad. 5, (2021).
- Kosari, A. et al. Cross-sectional characterization of the conversion layer formed on AA2024-T3 by a lithium-leaching coating. Appl. Surf. Sci. 512, 145665 (2020).
- Visser, P., Liu, Y., Terryn, H. & Mol, J. M. C. Lithium salts as leachable corrosion inhibitors and potential replacement for hexavalent chromium in organic coatings for the protection of aluminum alloys. J. Coatings Technol. Res. 13, 557–566 (2016).
- Marcoen, K. et al. Compositional study of a corrosion protective layer formed by leachable lithium salts in a coating defect on AA2024-T3 aluminium alloys. Prog. Org. Coatings 119, 65–75 (2018).
- Li, Z. et al. The Effect of Ambient Ageing on the Corrosion Protective Properties of a Lithium-Based Conversion Layer. J. Electrochem. Soc. 170, 031504 (2023).
- Visser, P. et al. The chemical throwing power of lithium-based inhibitors from organic coatings on AA2024-T3. Corros. Sci. 150, 194–206 (2019).
- Laird, J. S. et al. Particle induced gamma and X-ray emission spectroscopies of lithium based alloy coatings. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. with Mater. Atoms 404, 167–172 (2017).
- Visser, P. et al. Li leaching from Li carbonate-primer: Transport pathway development from the scribe edge of a primer/topcoat system. Prog. Org. Coatings 158, 106284 (2021).
- Hughes, A. et al. Particle characterisation and depletion of Li 2 CO 3 inhibitor in a polyurethane coating. Coatings 7, (2017).
- Dr. Peter Visser – Passion for paint – the science behind product development. 2023 (Young EFC – YouTube).
- Pereira, D. A. & Williams, J. A. Origin and evolution of high throughput screening. Br. J. Pharmacol. 152, 53–61 (2007).
- García, S. J. et al. The influence of pH on corrosion inhibitor selection for 2024-T3 aluminium alloy assessed by high-throughput multielectrode and potentiodynamic testing. Electrochim. Acta 55, 2457–2465 (2010).
- Muster, T. H. et al. A rapid screening multi-electrode method for the evaluation of corrosion inhibitors. Electrochim. Acta 54, 3402–3411 (2009).
- White, P. A. et al. A new high-throughput method for corrosion testing. Corros. Sci. 58, 327–331 (2012).
- White, P. A. et al. High-throughput channel arrays for inhibitor testing: Proof of concept for AA2024-T3. Corros. Sci. 51, 2279–2290 (2009).
- Winkler, D. A. et al. Using high throughput experimental data and in silico models to discover alternatives to toxic chromate corrosion inhibitors. Corros. Sci. 106, 229–235 (2016).
- Harvey, T. G. et al. The effect of inhibitor structure on the corrosion of AA2024 and AA7075. Corros. Sci. 53, 2184–2190 (2011).
- Taheri, P. et al. On the importance of time-resolved electrochemical evaluation in corrosion inhibitor-screening studies. npj Mater. Degrad. 4, 12 (2020).
- Coelho, L. B. et al. Reviewing machine learning of corrosion prediction in a data-oriented perspective. npj Mater. Degrad. 6, (2022).
- Himanen, L. et al. DScribe: Library of descriptors for machine learning in materials science. Comput. Phys. Commun. 247, (2020).
- MacLeod, B. P. et al. Self-driving laboratory for accelerated discovery of thin-film materials. Sci. Adv. 6, (2020).
Author Details
J. M. C. (Arjan) Mol is Professor Corrosion Technology and Electrochemistry at Delft University of Technology and he is Scientific Director of the 4TU.High-Tech Materials Centre in the Netherlands. The specific research focus areas of Mol embrace local corrosion, surface treatment, adhesive bonding, green inhibitors, active protective and self-healing coatings. Since 2017 he is Editor-in-Chief of Elsevier’s Corrosion Science and has served the European Federation of Corrosion in various leading positions. He received the European Corrosion Medal in 2022.
C. (Can) Özkan is a PhD candidate at Delft University of Technology and also serves as the Scientific Communication Officer board member for the young branch of the European Federation of Corrosion. His primary research focus is to enhance the understanding of corrosion inhibition and self-healing mechanisms within active protective aerospace coatings. His research interests lie at the intersection of electrochemistry, spectroscopy, and machine learning.