Authors: A. Lamin, A. H. Kaksonen, I. Cole, P. White and X.B. Chen,
This paper was presented at the Corrosion & Prevention 2023.
ABSTRACT
Microbiologically influenced corrosion (MIC) is a process in which microorganisms initiate, facilitate, and/or accelerate the corrosion reactions of metallic components. It is documented that MIC accounts for about 20 – 40 % of the total cost of corrosion. Biofilm formation on the surface of metal components plays a vital role in MIC, which leads to severe consequences in various environmental and industrial settings. Quorum sensing (QS) system is a key contributor to biofilm formation and the expression of some microbial enzymes. QS is a communication mechanism between microorganisms that involves the regulation of gene expression as a response to the microbial cell density within an environment. Both Gram-positive and Gram- negative bacteria employ it to regulate various physiological functions. QS involves production, and detection of, and responses to signalling chemicals, known as auto-inducers. QS controls specific processes important for the microbial community, such as biofilm formation, virulence factor expression, production of secondary metabolites and stress adaptation mechanisms. QS inhibitors (QSIs) has been proposed to address the biofilm related challenges in many different applications. Although QSIs have demonstrated some strength in tackling biofouling, QSI-based strategies to control microbially influenced corrosion have not been thoroughly investigated. As such, this article aims to target the QS mechanisms as a strategy for mitigating MIC on metal surfaces in engineered systems. Initial results obtained in this study confirmed QSI ability to slow the biofilm formation and increased the metal resistance to corrosion caused by Pseudomonas aeruginosa.
Keywords: quorum sensing, quorum quenching, biofilm, microbial influenced corrosion
Introduction
Quorum sensing (QS) is a mechanism that facilitates microbial communication. In this process bacteria produce and secrete low molecular weight signalling molecules, known as autoinducers (AIs), to their surroundings (Paluch et al. 2020). These diffusible molecules help bacteria to sense their population densities (Antunes et al. 2010; Paluch et al. 2020). At low cell density, AIs diffuse away, and, therefore, can’t be detected as they are present at concentrations below the threshold required for detection. When the bacterial density is high enough, AIs reach a threshold concentration and begin to bind to a receptor protein to activate various genes transcription. There are several different QS signalling systems employed by bacteria, but overall, the mechanism of QS remains consistent through all prokaryotes.
In general QS signalling can be grouped into two categories (Dalwadi & Pearce 2021). Most Gram-negative bacteria utilize N -acetyl- l -homoserine lactone (AHL) as signal molecules, while cell-to-cell signalling in Gram-positive bacteria uses oligopeptides signals (Zhou et al. 2020). QS process promotes the transcription of several genes, which regulate different vital bacterial processes such as bioluminescence, virulence factor production, sporulation, and biofilm formation. Biofilm formation is a defence mechanism used by microbes to survive stressful conditions. Sessile cells within biofilms are 100 times more resistant to harsh conditions (e.g., antibiotics, drugs, and heavy metals) compared to their planktonic counterparts. Biofilms can be problematic in a wide range of industrial fields. The development of biofilms on metal surfaces and the activities of bacteria beneath the film can lead to metal deterioration through microbially induced corrosion (MIC).
MIC is a process in which microorganisms initiate, facilitate, or accelerate the electrochemical corrosion reactions. Several reports documented that MIC accounts for about 20 to 40 % of the total cost of corrosion (Hashemi et al. 2018; Little et al. 2020). Microbes alter the electrochemical reaction at the biofilm/metal interface and either inhibit or accelerate the process of metal corrosion (Pal & Lavanya 2022). This concerning process usually involves the stimulation of microorganisms to cathodic or anodic processes or the creation of differential oxygen concentration cells in a confined electrolytic environment by microorganisms (Pal & Lavanya 2022).
Biofilms plays a crucial role in increasing corrosion at biological metal–solution interface, and it can happen in different ways, including (a) modifications in the transport of chemical species towards the metal surface; (b) generating uneven aeration effect because of the biofilm’s uneven distribution; (c) changing oxidation–reduction conditions at the metal–solution interface.; and (d) changing the structure of passive layers on metal surfaces. The metabolic activities of bacteria within the biofilm may have a significant impact on the MIC (Rao & Mulky 2023). In biofilms, microbes develop complicated interactions and become more resistant to biocides. Considering that there is no biofilm early monitoring system, and that biofouling is generally detected when losses in process performance or product quality happen, it is of great importance to slow or prevent their formation (Muhammad et al. 2020).
To understand the processes causing surfaces biofouling and MIC, it is crucial to take in consideration the basics of biofilm formation and development. Since bacterial biofilm formation is regulated by QS system, targeting biofilms through QSI’s will possibly control microbially induced corrosion on the metal surface. In this study, biofilm formation was studied on steel X65 using Laurie Britani media in the presence of the cinnamaldehyde QSI through electrochemical tests and scanning electron microscopy (SEM) analyses.
Materials and Methods
Sample Preparation
All specimens used in this work were X65 carbon steel with the elemental composition (wt.%) of 0.03 C, 0.17 Si,
1.51 Mn, 0.02 P,0.17 Ni, 0.04Cu, 0.16 Mo, 0.06 Nb, 0.02 Al, 0.01Ti and Fe and exposed surface area of 1 cm2. The work face of the specimens was abraded with 500, 800 and 1200 SiC paper and polished with diamond suspension to a 1 μm surface finish. Then degreased by anhydrous ethanol and was then dried in N2. All specimens were cleaned with
100% ethanol in an ultrasonic bath for 10 min and dried in a biosafety cabinet under ultraviolet (UV) light for 20- 30 min before use to minimise contamination.
Bacterial Culture and Corrosion experiment
Pseudomonas aeruginosa (ATCC 27853) was used for the experiments. The strain was initially sub-cultured aerobically three times to gain stable growth in the Luria-Bertani (LB) medium, which contained 10 g tryptone, 5 g yeast extract, and 10 g NaCl in 1000 mL deionized water. The optical density (O. D) of the bacterial culture was adjusted to 0.1 and a 2 ml of the inoculum culture was added to the LB solution in each corrosion cell. For electrochemical measurements, a standard three-electrode flat cell with an integrated platinum mesh counter electrode was used with a saturated calomel reference electrode. The polished steel samples were attached to the test cell as the working electrode with 0.78 cm2 exposed working area Every experiment was done with three replicates. The concentration of QSI was adjusted to 3 mM using a cinnamaldehyde DMSO solution of 1,510 mM.
Surface Analysis
Initial bacterial cell attachment and biofilm formation on steel X65 coupon surfaces was visualised using scanning electron microscopy (SEM) after 4 h, 8 h, 12 h, 7 d and 14 d incubation in LB broth and LB+3 mM cinnamaldehyde inoculated with P. aeruginosa. Prior to imaging, the samples were gently rinsed twice with phosphate-buffered saline (PBS, pH 7.4) and fixed with 2.5% glutaraldehyde for 30 min. The samples were then dehydrated using ethanol in 50, 70, 90, 95 and 100% concentrations and imaged using FEI Quanta 200 scanning electron microscopy.
Electrochemical Measurements
Electrochemical analysis was used to monitor the change in the electrochemical response at distinct stages of bacterial attachment and early biofilm life. For potentiodynamic polarisation scan (PDS) measurements, a standard three-electrode flat cell with an integrated platinum mesh counter electrode was used with a saturated calomel reference electrode (SCE). The polished steel samples were attached to the test cell as the working electrode with
0.78 cm2 exposed working area. With the test cell connected to a BioLogic®VMP-300 potentiostat, the test medium used was Luria Bertani poured into the test cell and the measured open circuit potential (OCP) was allowed to settle for various times. PDS tests were performed from -0.2 to 0.3 vs. OCP at 0.5 mV/sec after OCP. Three-electrode system was used in the test. The working electrode was X65 steel, the counter electrode was platinum mesh electrode, and the reference electrode was saturated calomel electrode. Firstly, OCP was measured, then electrochemical impedance spectroscopy (EIS) test was operated. EIS spectra that are used to determine the corrosion resistance were collected over the 1−20 MHz frequency range using a sinus amplitude of ±50 mVOCP.
Results and Discussion
PDS RESULTS
The results from the PDS studies are shown in Figure 1 and Table 1. The lowest icorr value after 12 h was obtained with the abiotic condition. When cinnamaldehyde was introduced to the biotic system there was decrease in the icorr value, while when cinnamaldehyde was introduced to the abiotic system there was an increase in icorr value. In general, this was the trend noticed in most of the time intervals chosen for PDS study which indicates that cinnamaldehyde might have a corrosive effect on steel X65. Interestingly, cinnamaldehyde showed different effect when it was used in the biotic system. This requires more investigation to understand the mechanism of reaction between the steel surface and the inhibitor and the bacteria with the inhibitor. Also, to confirm the effect of QS inhibitors, other inhibitors will be used in this study with the use of other culture media like minimal media to minimise the effect of media yeast extract on the corrosion process.
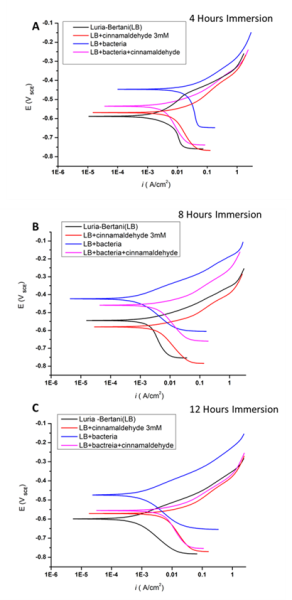
Figure 1. PDS results for steel X65 in four conditions: LB media, LB media+ QSI, LB media with P. aeruginosa, LB media + P. aeruginosa + QSI after A) 4 h, B) 8 h and C) 12 h incubation.
Table 1: Key electrochemical parameters estimated for steel X65 through Tafel fitting PDS plots after 4 h, 8 h and 12 h incubation in Luria-Bertani (LB) test media in the presence and absence of cinnamaldehyde quorum sensing inhibitor (QSI) and Pseudomonas aeruginosa bacteria.
| Incubation time (h) | Test media | Ecorr (mVSCE) | icorr (µA) | βC (mV/dec) | βA (mV/dec) |
| 4 | LB | -553±5 | 2.57±0.6 | 55.4 | 20.4 |
| LB+QSI | -578±2.5 | 1.15 ±0.4 | 41.4 | 16.6 | |
| LB+Bacteria | -535±4 | 4.22±0.5 | 517.4 | 56.7 | |
| LB+Bacteria+QSI | -568±5 | 0.89±0.5 | 21.4 | 12.8 | |
| 8 | LB | -544±1 | 0.88±0.6 | 93.9 | 35.6 |
| LB+QSI | -578±6 | 1.15±0.4 | 76.2 | 31.5 | |
| LB+Bacteria | -424±5 | 0.29±0.6 | 49.6 | 45.2 | |
| LB+Bacteria+QSI | -458±4 | 0.51±0.5 | 19.2 | 13.8 | |
| 12 | LB | -458±6 | 0.6±0.4 | 21.5 | 15.6 |
| LB+QSI | -569±5 | 1.11±0.5 | 31.5 | 14.4 | |
| LB+ Bacteria | -472±4 | 0.15±0.4 | 22.8 | 19.5 | |
| LB+Bacteria+QSI | -554±6 | 0.83±0.4 | 25.7 | 14 |
Electrochemical Impedance Spectroscopy (EIS) results
The results of steel X65 exposed to the QSI-amended system and non-amended systems after 3, 7, and 14 d were recorded using EIS and the day 7 results were as shown in Nyquist plot (Figure 2). Overall, Nyquist plot showed increasing semicircle diameter with time (not shown) which indicated increased surface resistance of the steel surface. The QSI supplementation to the LB medium may have slowed down the formation of this resistance layer as indicated by the small semicircle diameter as compared to unamended LB. In case of LB inoculated with bacteria, a biofilm developed, and it was initially protective to the surface. However, over time it became less protective as previously reported in the literature as different oxygen levels are created underneath the bacterial biofilm as it matures. In inoculated LB amended with QSI, the QSI may have slowed down the formation of the bacterial biofilm on the surface. More analysis is needed to EIS results using equivalent circuit to understand the different systems.
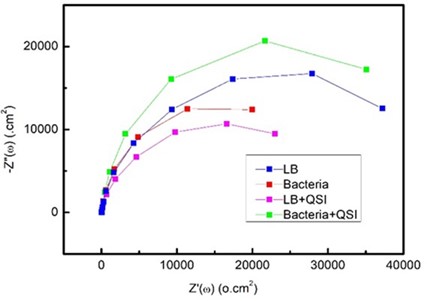
Figure2. Nyquist plot for the samples incubated for 7 d under different conditions with (LB) broth, LB with cinnamaldehyde quorum sensing inhibitor (QSI), LB with bacteria, LB with bacteria and QSI.
Surface Analysis
Initial cell attachment and biofilm formation by P. aeruginosa on steel X65 coupon surfaces as detected by SEM after 4 h, 8 h, 12 h, 7d and 14 d in LB broth and LB+3 mM cinnamaldehyde QSI are shown in Figure 3. The SEM images showed that in the early stages of biofilm formation (4-12 h) cinnamaldehyde QSI may have influenced the bacterial attachment to the steel surface. The system unamended with QSI showed aggregation of bacterial cells, and after 4 h in the culture medium, the P. aeruginosa biofilm started to form on the specimen surface, but on a few locations only, while in the QSI-amended system no cell aggregation was detected. After 7 days of incubation in the test solution (Figures 3g and 3f) a layer of compact biofilm was formed on the specimen surface, and P. aeruginosa cells were clearly visible in Fig. 3g. It has been suggested in literature that a compact biofilm could offer protection against corrosion which might explain some of the results obtained from electrochemistry tests (Kip & Van Veen 2015).. Literature indicates that QSIs can lead to smaller number of cells attached to the surface which results in limited biofilm formation and easier eradication of biofilms. The SEM images showed that biofilm coverage to steel surface generally increased with increasing the incubation time. This was likely due to the increase in the numbers of planktonic bacteria in the test media due to growth which subsequently caused an increase in the number of sessile bacteria attaching on the coupon surface with time. After 7 days of incubation in the presence of the QSI a flat and less compact biofilm and a smaller number of cells were detected on the surface. However, a total cell count is still needed to confirm the results obtained from SEM.

Figure 3. Scanning electron microscopy images showing early stages of Pseudomonas aeruginosa biofilm formation on steel X65 coupon surfaces for after 4 h (a,b), 8 h (c,d), 12 h (e,f), 7d (g,h) and 14 d (I,j) in LB broth (a, c, e, g and I, respectively) and LB+3 mM cinnamaldehyde quorum sensing inhibitor (QSI) (b, d, f, h and j, respectively).
Conclusions
This study investigated the attachment of P. aeruginosa and subsequent MIC of steel X65 coupons with and without the addition of cinnamaldehyde QSI to the test medium. The overall conclusions were:
- Differences were seen in the extent of the initial attachment of aeruginosa as well as the type of biofilm that formed on the surface depending on whether cinnamaldehyde was added.
- The initial corrosion study of the potential QSI showed that the use of certain inhibitors can have unintended consequences in relation to MIC (e.g., be more corrosive in abiotic conditions).
- The addition of QSI did not show any notable difference in the short-term (7 days) of biotic and abiotic The QSI might have another mechanism in affecting MIC rather than the biofilm inhibition, as SEM images showed that even in the presence of the QSI biofilm started to form after 7 days of incubation. To try and draw a direct relationship between QS inhibition and corrosion mitigation, other inhibitors and growth media will be used in this study.
In general, this study provided interesting results and conclusions that were not expected, especially that the culture media used in the system had a protective effect against corrosion during the first 7 days. This shows the importance of considering the choice of culture media and the effect it has on MIC, as a different culture media might give different results even if the same bacteria and QSI were used. Future work in this study will involve using growth media that can support bacterial growth but has minimal effect on metal like the minimal media that is widely used in MIC studies.
Acknowledgments
This research was supported by the Australian government and RMIT University.
References
Antunes, LCM, Ferreira, RBR, Buckner, MMC & Finlay, BB 2010, ‘Quorum sensing in bacterial virulence’, Microbiology, vol. 156, no. Pt 8, pp. 2271-2282.
Dalwadi, MP & Pearce, P 2021, ‘Emergent robustness of bacterial quorum sensing in fluid flow’, Proceedings of the National Academy of Sciences, vol. 118, no. 10, p. e2022312118.
Paluch, E, Rewak-Soroczyńska, J, Jędrusik, I, Mazurkiewicz, E & Jermakow, K 2020, ‘Prevention of biofilm formation by quorum quenching’, Appl Microbiol Biotechnol, vol. 104, no. 5, pp. 1871-1881.
Antunes, LCM, Ferreira, RBR, Buckner, MMC & Finlay, BB 2010, ‘Quorum sensing in bacterial virulence’, Microbiology, vol. 156, no. Pt 8, pp. 2271-2282.
Dalwadi, MP & Pearce, P 2021, ‘Emergent robustness of bacterial quorum sensing in fluid flow’, Proceedings of the National Academy of Sciences, vol. 118, no. 10, p. e2022312118.
Hashemi, SJ, Bak, N, Khan, F, Hawboldt, K, Lefsrud, L & Wolodko, J 2018, ‘Bibliometric analysis of microbiologically influenced corrosion (MIC) of oil and gas engineering systems’, Corrosion, vol. 74, no. 4, pp. 468-486.
Little, BJ, Blackwood, DJ, Hinks, J, Lauro, FM, Marsili, E, Okamoto, A, Rice, SA, Wade, SA & Flemming, HC 2020, ‘Microbially influenced corrosion—any progress?’, Corrosion Science, vol. 170, p. 108641.
Paluch, E, Rewak-Soroczyńska, J, Jędrusik, I, Mazurkiewicz, E & Jermakow, K 2020, ‘Prevention of biofilm formation by quorum quenching’, Appl Microbiol Biotechnol, vol. 104, no. 5, pp. 1871-1881.
Zhou, L, Zhang, Y, Ge, Y, Zhu, X & Pan, J 2020, ‘Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation’, Frontiers in microbiology, vol. 11, p. 589640.
Antunes, LCM, Ferreira, RBR, Buckner, MMC & Finlay, BB 2010, ‘Quorum sensing in bacterial virulence’, Microbiology, vol. 156, no. Pt 8, pp. 2271-2282.
Dalwadi, MP & Pearce, P 2021, ‘Emergent robustness of bacterial quorum sensing in fluid flow’, Proceedings of the National Academy of Sciences, vol. 118, no. 10, p. e2022312118.
Hashemi, SJ, Bak, N, Khan, F, Hawboldt, K, Lefsrud, L & Wolodko, J 2018, ‘Bibliometric analysis of microbiologically influenced corrosion (MIC) of oil and gas engineering systems’, Corrosion, vol. 74, no. 4, pp. 468-486.
Little, BJ, Blackwood, DJ, Hinks, J, Lauro, FM, Marsili, E, Okamoto, A, Rice, SA, Wade, SA & Flemming, HC 2020, ‘Microbially influenced corrosion—any progress?’, Corrosion Science, vol. 170, p. 108641.
Pal, MK & Lavanya, M 2022, ‘Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation’, Journal of Bio- and Tribo-Corrosion, vol. 8, no. 3.
Paluch, E, Rewak-Soroczyńska, J, Jędrusik, I, Mazurkiewicz, E & Jermakow, K 2020, ‘Prevention of biofilm formation by quorum quenching’, Appl Microbiol Biotechnol, vol. 104, no. 5, pp. 1871-1881.
Rao, P & Mulky, L 2023, ‘Microbially Influenced Corrosion and its Control Measures: A Critical Review’, Journal of Bio-and Tribo-Corrosion, vol. 9, no. 3, p. 57.
Zhou, L, Zhang, Y, Ge, Y, Zhu, X & Pan, J 2020, ‘Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation’, Frontiers in microbiology, vol. 11, p. 589640.
Antunes, LCM, Ferreira, RBR, Buckner, MMC & Finlay, BB 2010, ‘Quorum sensing in bacterial virulence’, Microbiology, vol. 156, no. Pt 8, pp. 2271-2282.
Dalwadi, MP & Pearce, P 2021, ‘Emergent robustness of bacterial quorum sensing in fluid flow’, Proceedings of the National Academy of Sciences, vol. 118, no. 10, p. e2022312118.
Hashemi, SJ, Bak, N, Khan, F, Hawboldt, K, Lefsrud, L & Wolodko, J 2018, ‘Bibliometric analysis of microbiologically influenced corrosion (MIC) of oil and gas engineering systems’, Corrosion, vol. 74, no. 4, pp. 468-486.
Little, BJ, Blackwood, DJ, Hinks, J, Lauro, FM, Marsili, E, Okamoto, A, Rice, SA, Wade, SA & Flemming, HC 2020, ‘Microbially influenced corrosion—any progress?’, Corrosion Science, vol. 170, p. 108641.
Muhammad, MH, Idris, AL, Fan, X, Guo, Y, Yu, Y, Jin, X, Qiu, J, Guan, X & Huang, T 2020, ‘Beyond risk: bacterial biofilms and their regulating approaches’, Frontiers in microbiology, vol. 11, p. 928.
Pal, MK & Lavanya, M 2022, ‘Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation’, Journal of Bio- and Tribo-Corrosion, vol. 8, no. 3.
Paluch, E, Rewak-Soroczyńska, J, Jędrusik, I, Mazurkiewicz, E & Jermakow, K 2020, ‘Prevention of biofilm formation by quorum quenching’, Appl Microbiol Biotechnol, vol. 104, no. 5, pp. 1871-1881.
Rao, P & Mulky, L 2023, ‘Microbially Influenced Corrosion and its Control Measures: A Critical Review’, Journal of Bio-and Tribo-Corrosion, vol. 9, no. 3, p. 57.
Zhou, L, Zhang, Y, Ge, Y, Zhu, X & Pan, J 2020, ‘Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation’, Frontiers in microbiology, vol. 11, p. 589640.
Author Details
A. Lamin
She is currently undertaking research as a PhD candidate at RMIT University, with a focus on the role of bacteria communication system on microbial corrosion. She has a background in biotechnology.
A. Kaksonen
She is a Group Leader for Industrial Biotechnology at the Commonwealth Scientific and Industrial Research Organisation (CSIRO). She develops biotechnological processes for environmental and industrial applications in the mining, energy, water supply, waste, and wastewater treatment industries. Her research topics have included: mining biotechnology, bioenergy, bioremediation of contaminated sites, waste/wastewater treatment and resource recovery, circular economy and mitigation of microbially caused problems such as biofouling, bio clogging and biocorrosion.
Ivan Cole
He is a Professor of Engineering at RMIT. Research interests are corrosion modelling and sensing and development of new inhibitors. His modelling work focuses on linking scales from the molecular to the continental to understand both the fine scale process and the factors controlling corrosion. His sensor work concentrates on AI to enhance sensor data interpretation while he is developing rapid discovery methods (for inhibitors) combining molecular modelling, AI and robotic electrochemistry.
Dr Paul White
He is a Principal Research Fellow at RMIT University. He has developed new high-throughput techniques for corrosion inhibition together with the data capture and data analysis of the resulting cavalcade of ensuing experimental data, resulting in various patents relating to corrosion inhibitor protection of aerospace aluminium alloys and steel.
Dr. Xiaobo Chen
He is Senior Lecturer at School of Engineering, RMIT University. His research expertise is multidisciplinary and spans from chemistry and materials science through to corrosion, electrochemistry, and biomaterials. Over the last decade, his endeavours have been aiming to provide functional characteristics upon surface of light metals to meet the requirements for a large range of engineering applications in automotive, 3C and biomedical industries.
Antunes, LCM, Ferreira, RBR, Buckner, MMC & Finlay, BB 2010, ‘Quorum sensing in bacterial virulence’,
Microbiology, vol. 156, no. Pt 8, pp. 2271-2282.
Dalwadi, MP & Pearce, P 2021, ‘Emergent robustness of bacterial quorum sensing in fluid flow’, Proceedings of the National Academy of Sciences, vol. 118, no. 10, p. e2022312118.
Hashemi, SJ, Bak, N, Khan, F, Hawboldt, K, Lefsrud, L & Wolodko, J 2018, ‘Bibliometric analysis of microbiologically influenced corrosion (MIC) of oil and gas engineering systems’, Corrosion, vol. 74, no. 4, pp. 468-486.
Kip, DJ & Van Veen, JA 2015, ‘The dual role of microbes in corrosion’, ISME J, vol. 9, no. 3, pp. 542-551.
Little, BJ, Blackwood, DJ, Hinks, J, Lauro, FM, Marsili, E, Okamoto, A, Rice, SA, Wade, SA & Flemming, HC 2020, ‘Microbially influenced corrosion—any progress?’, Corrosion Science, vol. 170, p. 108641.
Muhammad, MH, Idris, AL, Fan, X, Guo, Y, Yu, Y, Jin, X, Qiu, J, Guan, X & Huang, T 2020, ‘Beyond risk: bacterial biofilms and their regulating approaches’, Frontiers in microbiology, vol. 11, p. 928.
Pal, MK & Lavanya, M 2022, ‘Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation’,
Journal of Bio- and Tribo-Corrosion, vol. 8, no. 3.
Paluch, E, Rewak-Soroczyńska, J, Jędrusik, I, Mazurkiewicz, E & Jermakow, K 2020, ‘Prevention of biofilm formation by quorum quenching’, Appl Microbiol Biotechnol, vol. 104, no. 5, pp. 1871-1881.
Rao, P & Mulky, L 2023, ‘Microbially Influenced Corrosion and its Control Measures: A Critical Review’, Journal of Bio-and Tribo-Corrosion, vol. 9, no. 3, p. 57.
Zhou, L, Zhang, Y, Ge, Y, Zhu, X & Pan, J 2020, ‘Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation’, Frontiers in microbiology, vol. 11, p. 589640.